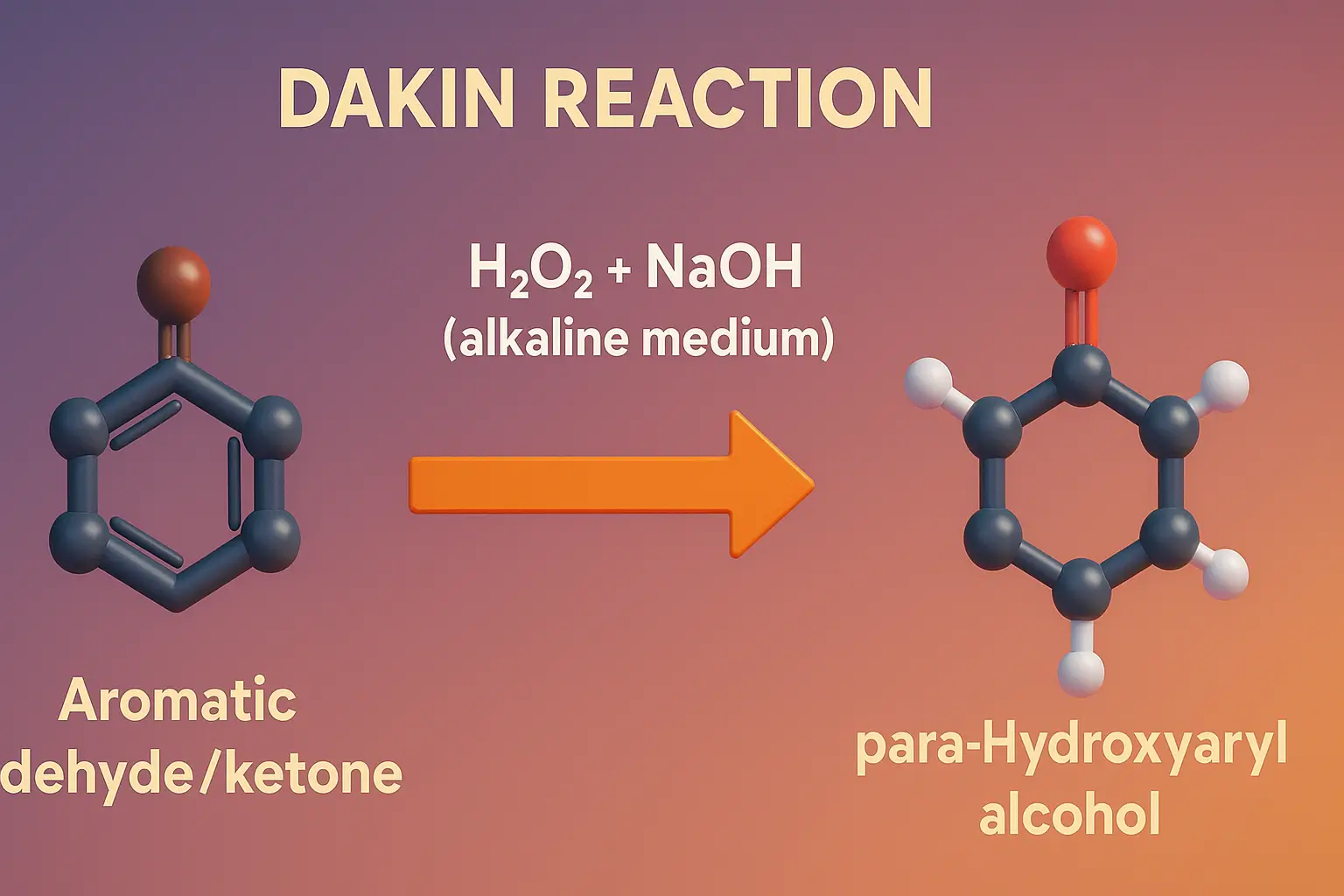
Dakin Reaction converts ortho- and para-hydroxy aromatic aldehydes or ketones to dihydroxybenzenes using hydrogen peroxide.
Overview of Dakin Reaction:
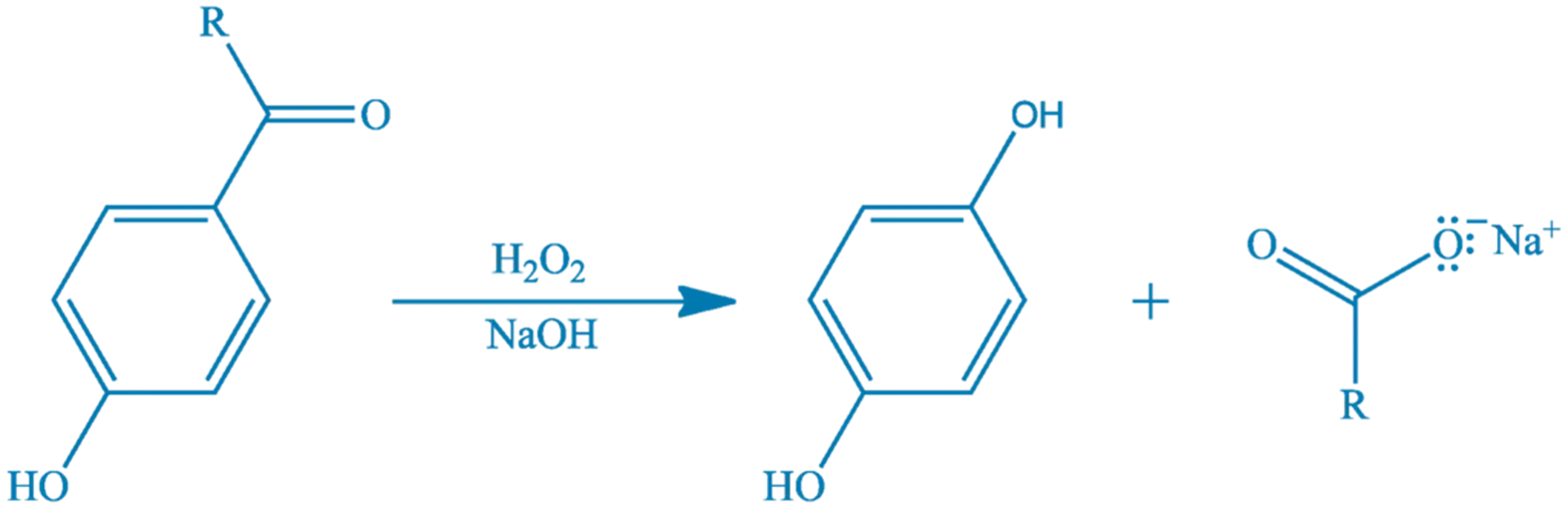
- The Dakin reaction involves the oxidation of aryl aldehydes or aryl ketones, especially those with electron-donating groups (–OH or –OR) in the ortho or para position, to phenols using hydrogen peroxide in basic medium.
Advertisements
Reagents:
- Hydrogen peroxide (H2O2)
- Base (e.g., NaOH or KOH)
- Aqueous or alcoholic medium
General Reaction:
For aryl aldehyde:
Ar–CHO + H2O2/NaOH → Ar–OH + HCOO⁻
Advertisements
For aryl ketone:
Ar–CO–R + H2O2/NaOH → Ar–OH + R–COO⁻
Advertisements
Mechanism of Dakin Reaction:
Step 1: Nucleophilic addition
- Under basic conditions, H2O2 forms the hydroperoxide anion (⁻OOH).
- This anion attacks the electrophilic carbonyl carbon to form a tetrahedral intermediate.
Step 2: Aryl migration
- The aryl group migrates to the adjacent oxygen (from the hydroperoxide group).
- A rearrangement occurs, and the leaving group (formate or carboxylate) departs.
Step 3: Hydrolysis
- The aryl peroxide formed is hydrolyzed to produce a phenol.
Advertisements
Key Features:
- Requires electron-donating substituents for activation
- Selective for ortho- and para-substituted aryl aldehydes and ketones
- Environmentally friendly (H2O2 is a green oxidant)
- Useful in synthetic and pharmaceutical chemistry