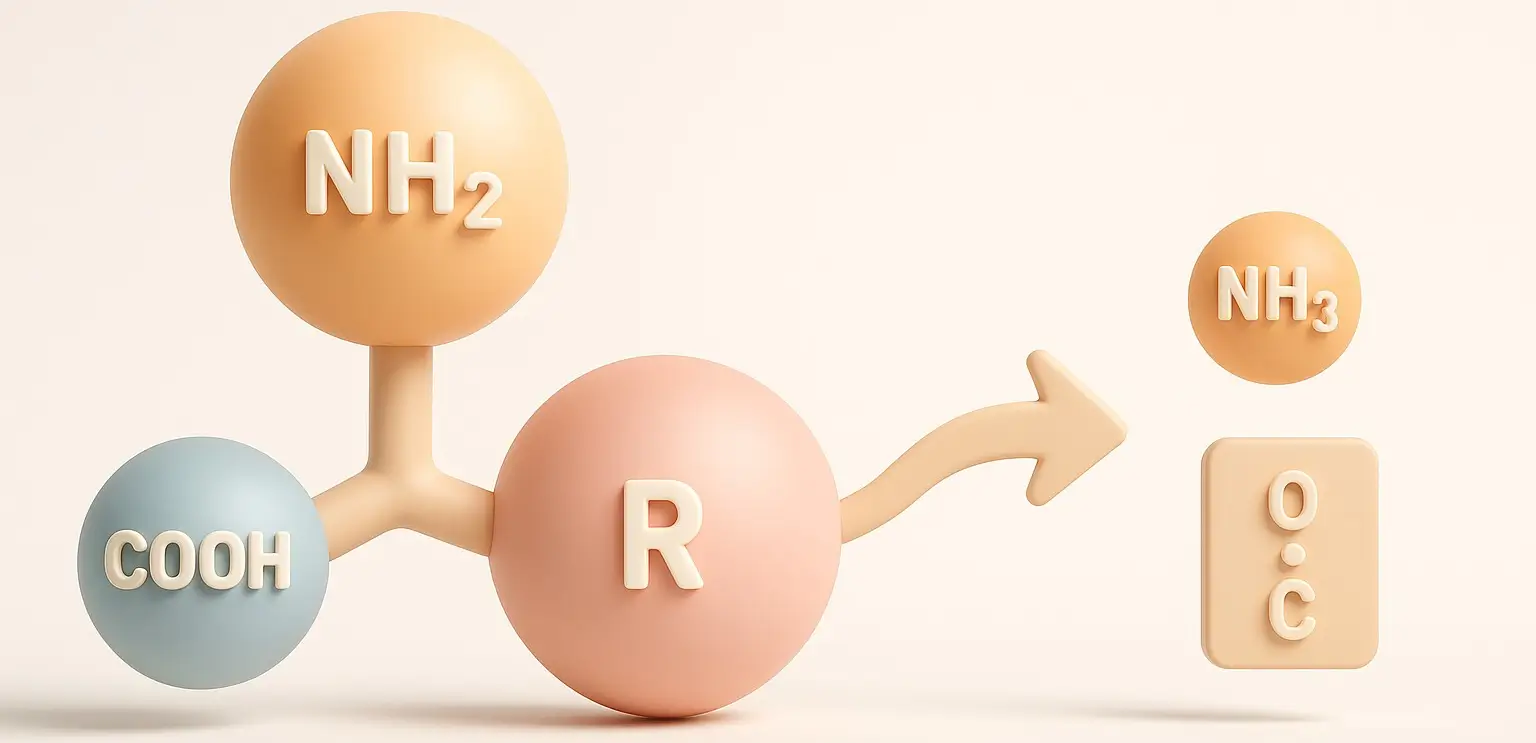- Deamination is an essential biochemical process that facilitates the removal of an amino group (-NH2) from an amino acid.
- This process yields ammonia (NH3) and an α-keto acid, playing a pivotal role in the catabolism (breakdown) of amino acids.
- Through deamination, the body can utilize amino acids for energy, produce metabolic intermediates, and manage excess nitrogen effectively.
Types of Deamination Reactions:
-
Oxidative Deamination:
- Mechanism: The amino group is removed through an oxidation process, producing an α-keto acid, ammonia, and a reduced cofactor (e.g., NADH).
- Enzymatic Catalysis: Dehydrogenases catalyze these reactions. A prominent example is the enzyme glutamate dehydrogenase, which converts glutamate into α-ketoglutarate, generating ammonia and NADH (or NADPH).
-
Non-oxidative Deamination:
- Mechanism: This involves initially transferring the amino group from the amino acid to a different molecule, typically via a transamination reaction. The amino group is then released as ammonia.
- Enzymatic Process: Occurs in a two-step enzymatic process:
- First Step: A transaminase enzyme transfers the amino group to another molecule.
- Second Step: The amino group is released as ammonia, often facilitated by enzymes such as glutamate dehydrogenase. For example, the deamination of alanine to pyruvate involves transferring alanine’s amino group to α-ketoglutarate, forming glutamate and pyruvate, followed by the release of ammonia from glutamate.
Ammonia Detoxification:
- The ammonia produced during deamination is toxic and must be detoxified.
- The urea cycle, predominantly occurring in the liver, plays a critical role in converting ammonia into urea, a less toxic compound.
- Urea is then safely excreted from the body through the kidneys.
Physiological Significance:
- Deamination is integral to amino acid metabolism, enabling the body to adapt to various energy demands and maintain nitrogen balance.
- It allows for the repurposing of amino acids as energy sources and the production of key metabolic intermediates.
Advertisements
Clinical Implications:
- Disruptions in deamination processes can lead to elevated ammonia levels, posing significant health risks.
- Such abnormalities can cause metabolic disorders and severe health complications due to ammonia’s toxicity.
- Monitoring and managing deamination processes are crucial for preventing these adverse effects and ensuring metabolic health.