- Scope of pharmaceutical analysis is a specialized branch of analytical chemistry focused on the identification, quantification, and determination of the quality, safety, and efficacy of pharmaceutical products.
- It involves the application of various analytical techniques and methodologies to ensure that drugs and other pharmaceutical substances adhere to established standards and regulatory requirements.
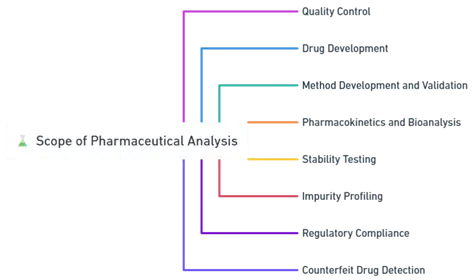
Scope of Pharmaceutical Analysis:
-
Quality Control:
- Ensures pharmaceutical products meet quality standards for purity, potency, stability, and uniformity.
- Involves analysis of raw materials, in-process materials, finished products, and packaging.
-
Drug Development:
- Supports the identification and characterization of APIs and excipients.
- Aids in optimizing formulations and manufacturing processes.
-
Method Development and Validation:
- Focuses on creating, optimizing, and validating analytical methods to ensure accuracy, precision, and reliability.
-
Pharmacokinetics and Bioanalysis:
- Analyzes drug ADME (absorption, distribution, metabolism, excretion) to guide dosing and understand pharmacological effects.
-
Stability Testing:
- Evaluates drug stability under various conditions to determine shelf life and proper storage.
-
Impurity Profiling:
- Identifies and quantifies impurities to ensure drug safety and efficacy.
-
Regulatory Compliance:
- Ensures adherence to regulations and guidelines from authorities like the FDA and EMA.
-
Counterfeit Drug Detection:
- Identifies counterfeit or adulterated drugs to safeguard public health and maintain the pharmaceutical supply chain.
Click Here to Watch the Best Pharma Videos
Advertisements