- Determination of Reaction Order defines how reactant concentration influences reaction rate.
- Determining the order of a degradation reaction involves monitoring concentration over time and fitting data to various integrated rate equations.
Methods of Determination of Reaction Order:
-
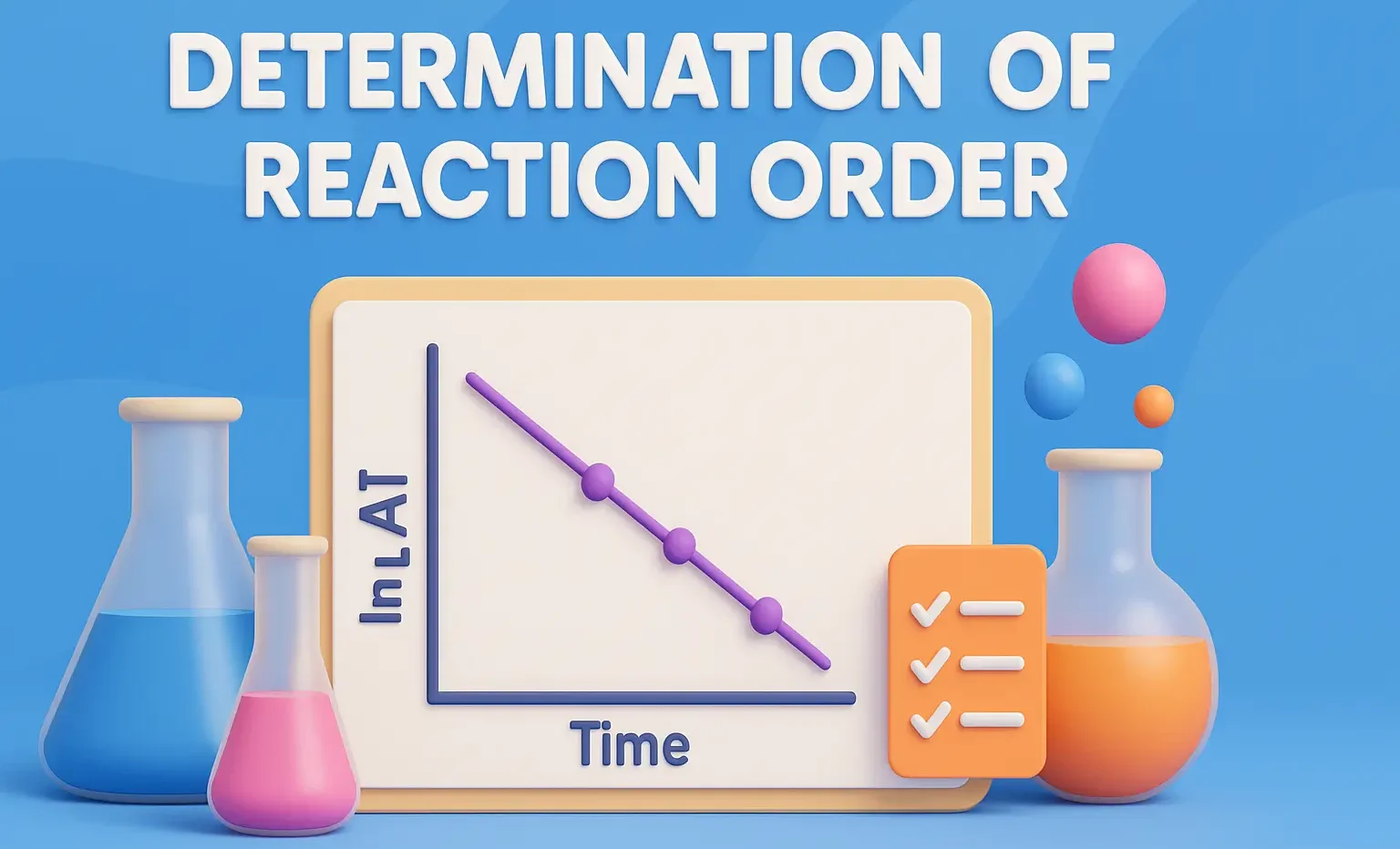
Graphical Method
- Plot concentration vs. time for different rate laws:
- Zero order: [A] vs. time (linear)
- First order: time (linear)
- Second order: time (linear)
- The one that gives a straight line indicates the reaction order.
- Plot concentration vs. time for different rate laws:
-
Method of Initial Rates
- Run multiple experiments with different initial concentrations.
- Measure the initial rate.
- Use:
$\text{Rate} = k[A]^n \;\;\Rightarrow\;\; \log(\text{Rate}) = \log(k) + n \log([A])$
-
- $\text{Plot } \log(\text{Rate}) \; \text{ vs. } \log([A]) \rightarrow \text{Slope} = n$
-
Half-Life Method
- Measure how half-life changes with initial concentration:
- Zero-order: t_{1/2} \propto [A]_0
- First-order: t_{1/2} = \text{constant}
- Second-order: t_{1/2} \propto \frac{1}{[A]_0}
- Measure how half-life changes with initial concentration:
-
Curve Fitting Software
- Use software (e.g., WinNonlin, Excel, MATLAB) to fit experimental data to kinetic models
Click Here to Watch the Best Pharma Videos
Advertisements