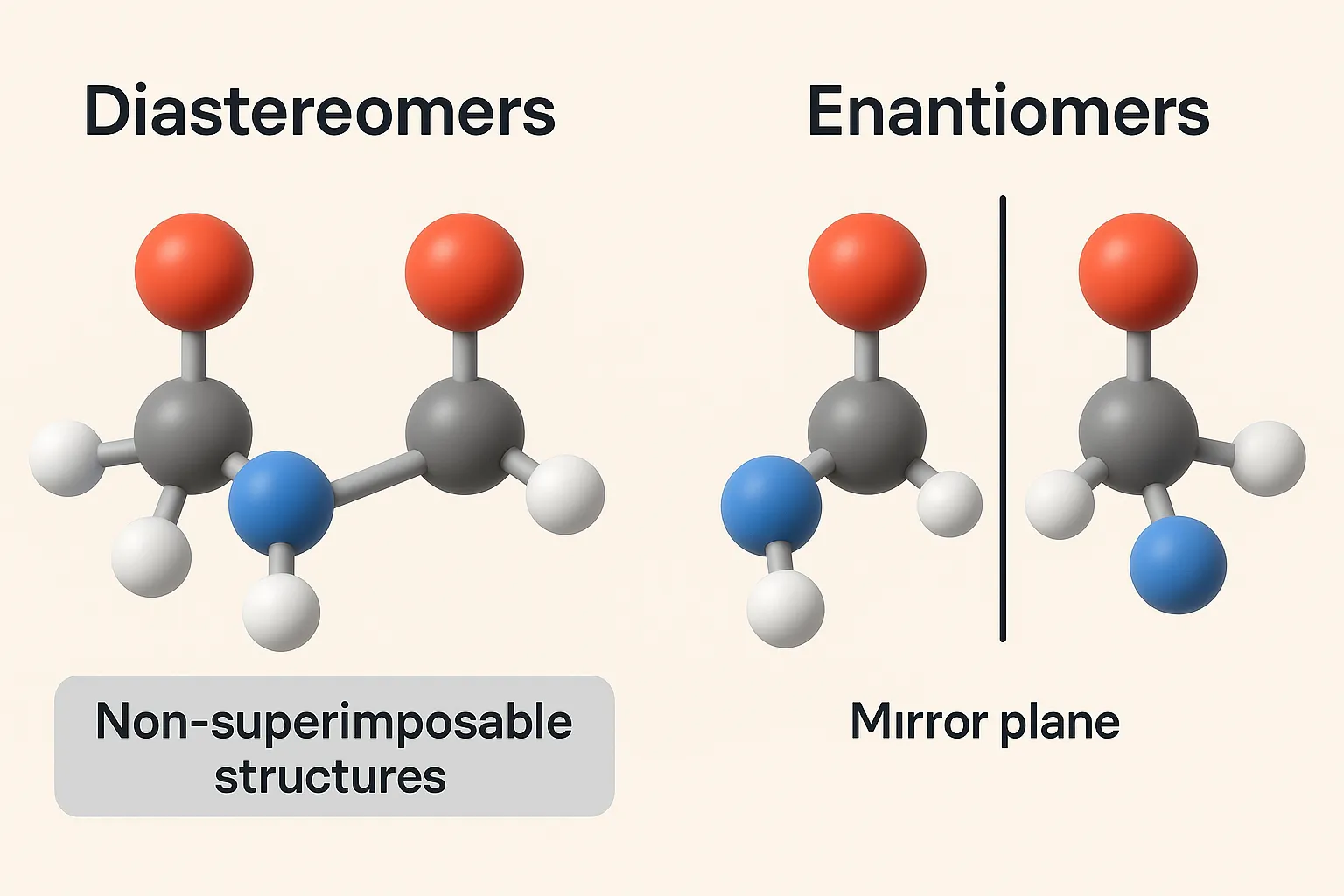
Diastereomerism is a type of stereoisomerism where molecules have the same molecular formula but are not mirror images of each other. Unlike enantiomers, diastereomers differ in physical and chemical properties such as melting point, solubility, and reactivity. This distinction makes diastereomerism important in drug design, as different diastereomers of the same compound may show varied biological activities and therapeutic effects.
Definition of the Diastereomerism:
- Diastereomers are stereoisomers that are not mirror images and not superimposable.
- They usually occur in molecules with more than one chiral center.
Key characteristics:
- Differ in physical and chemical properties
- May or may not be optically active
- They differ at some, but not all, chiral centers
Example of Diastereomerism:
- Tartaric acid (HOOC–CH(OH)–CH(OH)–COOH):
- Has two chiral centers.
- Possible stereoisomers:
- (R,R)
- (S,S)
- (R,S) — not a mirror image of the others, hence a diastereomer
Click Here to Watch the Best Pharma Videos