Learn about Diphenhydramine Hydrochloride, its uses as an antihistamine, mechanism of action, and role in allergy and cold treatment.
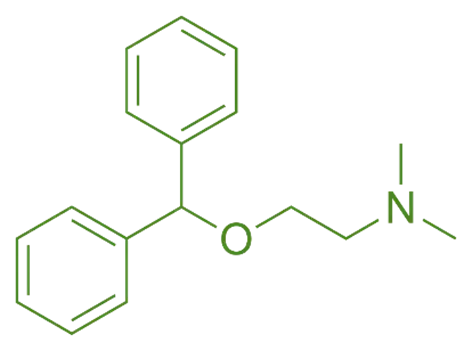
Structure
- Diphenhydramine hydrochloride is a first-generation antihistamine characterized by two phenyl rings connected by an ethylamine chain.
- The chemical structure includes:
- Two benzene rings (diphenyl)
- An ether linkage (-O-) between them
- An ethyl chain ending with a dimethylamino group
- Protonated hydrochloride salt form
- Chemical Formula: C₁₇H₂₁NO·HCl
Mode of Action
- Diphenhydramine acts as a competitive antagonist at H₁-receptors. I
- t crosses the blood-brain barrier, leading to central nervous system (CNS) effects such as sedation. Additionally, it exhibits anticholinergic and antiemetic properties.
Advertisements
Uses
- Allergic rhinitis
- Urticaria (hives)
- Common cold symptoms
- Motion sickness (in combination with other agents like in Dimenhydrinate)
- Insomnia
- Cough suppressant (in some formulations)
Structure-Activity Relationship (SAR)
- Aromatic Rings: The two phenyl rings enhance lipophilicity, facilitating crossing the blood-brain barrier, contributing to sedative effects.
- Ethylene Bridge: Provides the necessary flexibility for receptor binding.
- Dimethylamino Group: Essential for H₁ receptor antagonism; the basic nitrogen forms hydrogen bonds with the receptor.
- Hydroxyl Groups: Presence in related structures can modify lipophilicity and metabolism.
Synthesis
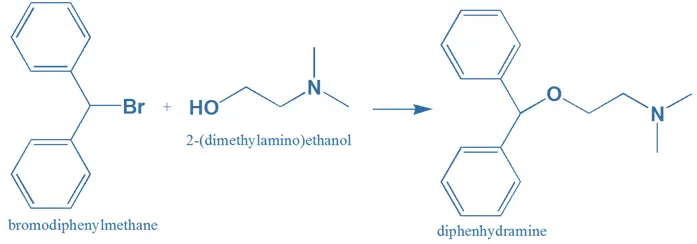
- Diphenylmethane reacts with Bromine (Bromination) in the presence of light to form Diphenylbromomethane.
- Diphenylbromomethane will react with Dimethylamino ethanol. (Bromine will take H⁺ molecule and goes out as HBr)
- Final product will form Diphenhydramine.
Click Here to Watch the Best Pharma Videos
Advertisements