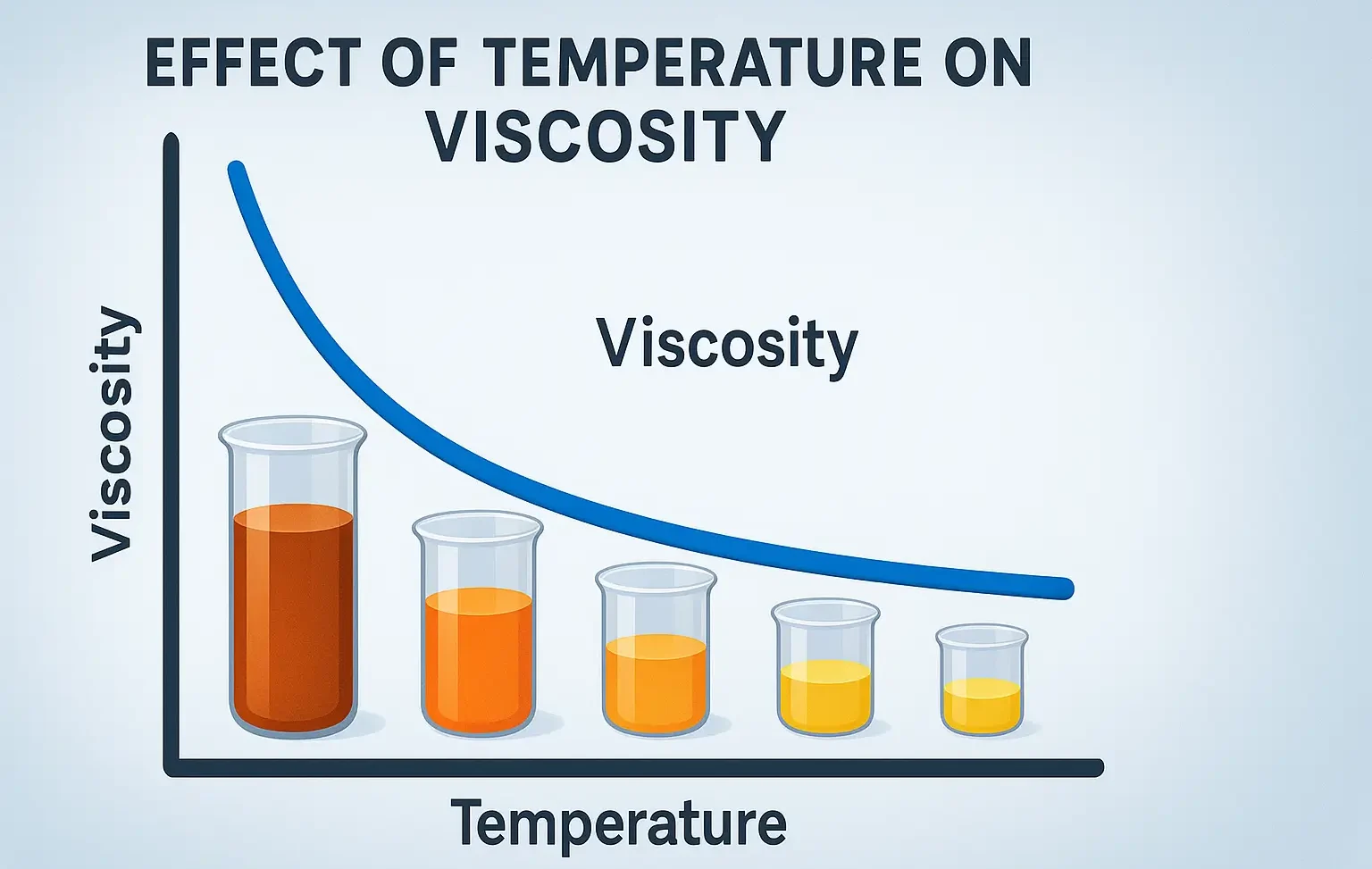
- Effect of Temperature on Viscosity is key in drug formulation, lubrication, and industrial processes.
- Effect of Temperature on Viscosity shows liquids thin as heat rises and molecules move faster.
- Viscosity is inversely related to temperature – as temperature increases, viscosity usually decreases.
Why?
- At higher temperatures, the kinetic energy of molecules increases.
- This reduces intermolecular forces (less resistance to flow).
- Hence, the fluid becomes less viscous (flows more easily).
Quantitative Expression:
- Viscosity changes with temperature can be described by the Arrhenius equation:
- $\eta = A \cdot e^{\tfrac{E}{RT}}$
Where:
Advertisements
- η = viscosity
- A = pre-exponential factor
- E = activation energy of flow
- R = gas constant
- T = temperature (Kelvin)