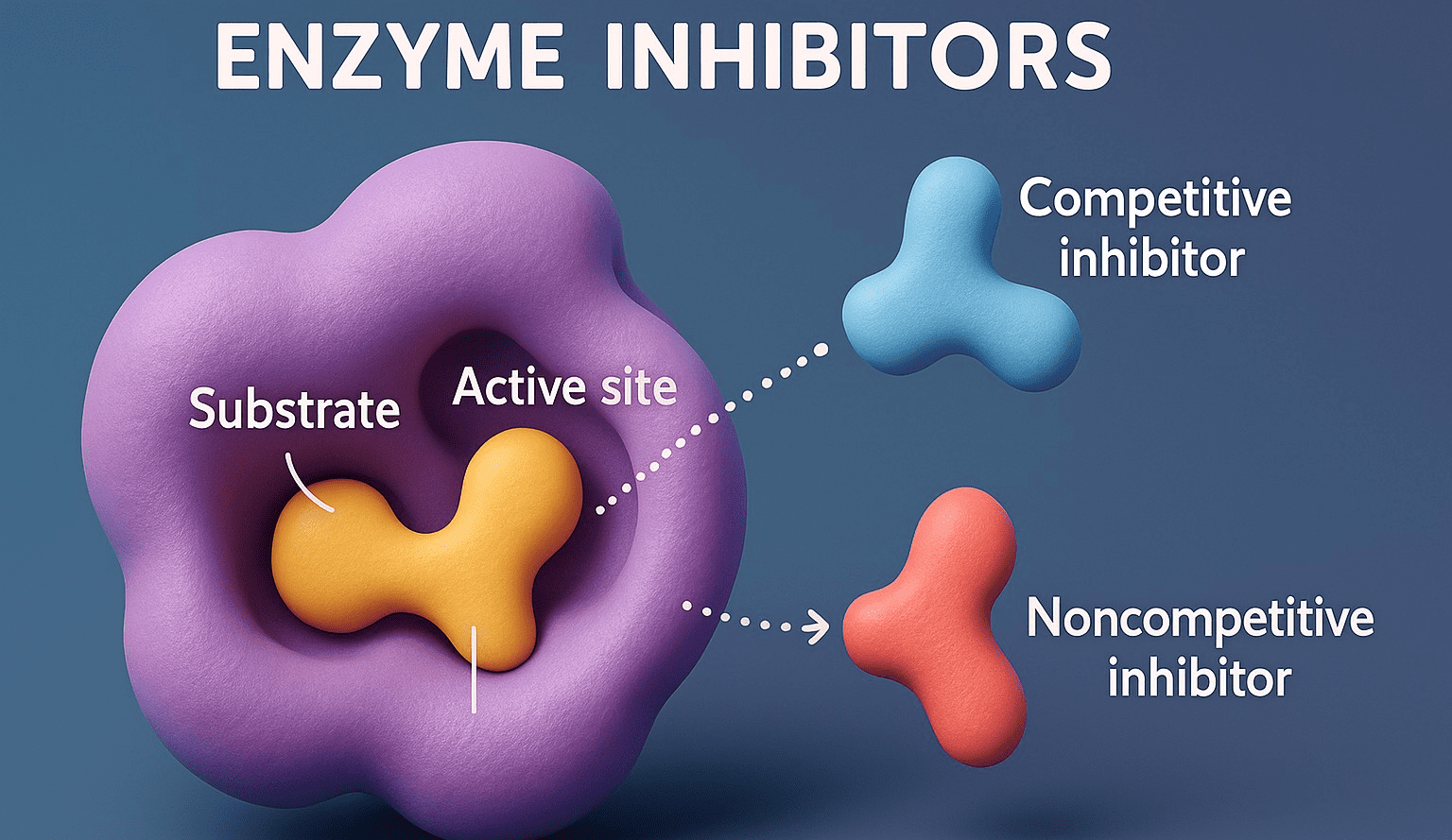
- Enzyme inhibitors are molecules that interact with enzymes to regulate their activities.
- They are vital for cellular control and pharmaceutical development of Enzyme Inhibitors, targeting specific enzymatic pathways to treat diseases.
- Inhibitors can be classified based on their reversibility and mode of action.
Reversible Enzyme Inhibitors
- Reversible inhibitors temporarily bind to enzymes and are classified into three types:
-
Competitive Enzyme Inhibitors
- Mechanism: Compete with the substrate for the active site due to structural similarity.
- Overcoming Inhibition: Increasing substrate concentration can reduce inhibition.
- Example: Methotrexate inhibits dihydrofolate reductase, used in cancer and autoimmune disease treatment.
-
Non-competitive Inhibitors
- Mechanism: Bind to an allosteric site, causing conformational changes that reduce enzyme function.
- Example: Allopurinol inhibits xanthine oxidase, reducing uric acid production to treat gout and prevent kidney stones.
-
Uncompetitive Inhibitors
- Mechanism: Bind exclusively to the enzyme-substrate complex, preventing product release.
- Example: Lithium ions inhibit inositol monophosphates, used in managing bipolar disorder.
Irreversible Inhibitors
- Irreversible inhibitors bind covalently to enzymes, causing permanent deactivation.
- They often target critical amino acids in the active site.
- Example: Aspirin irreversibly inhibits cyclooxygenase (COX) enzymes by acetylating a serine residue, blocking prostaglandin synthesis, and providing anti-inflammatory, analgesic, and antipyretic effects.
Thank you for reading from Firsthope's notes, don't forget to check YouTube videos!