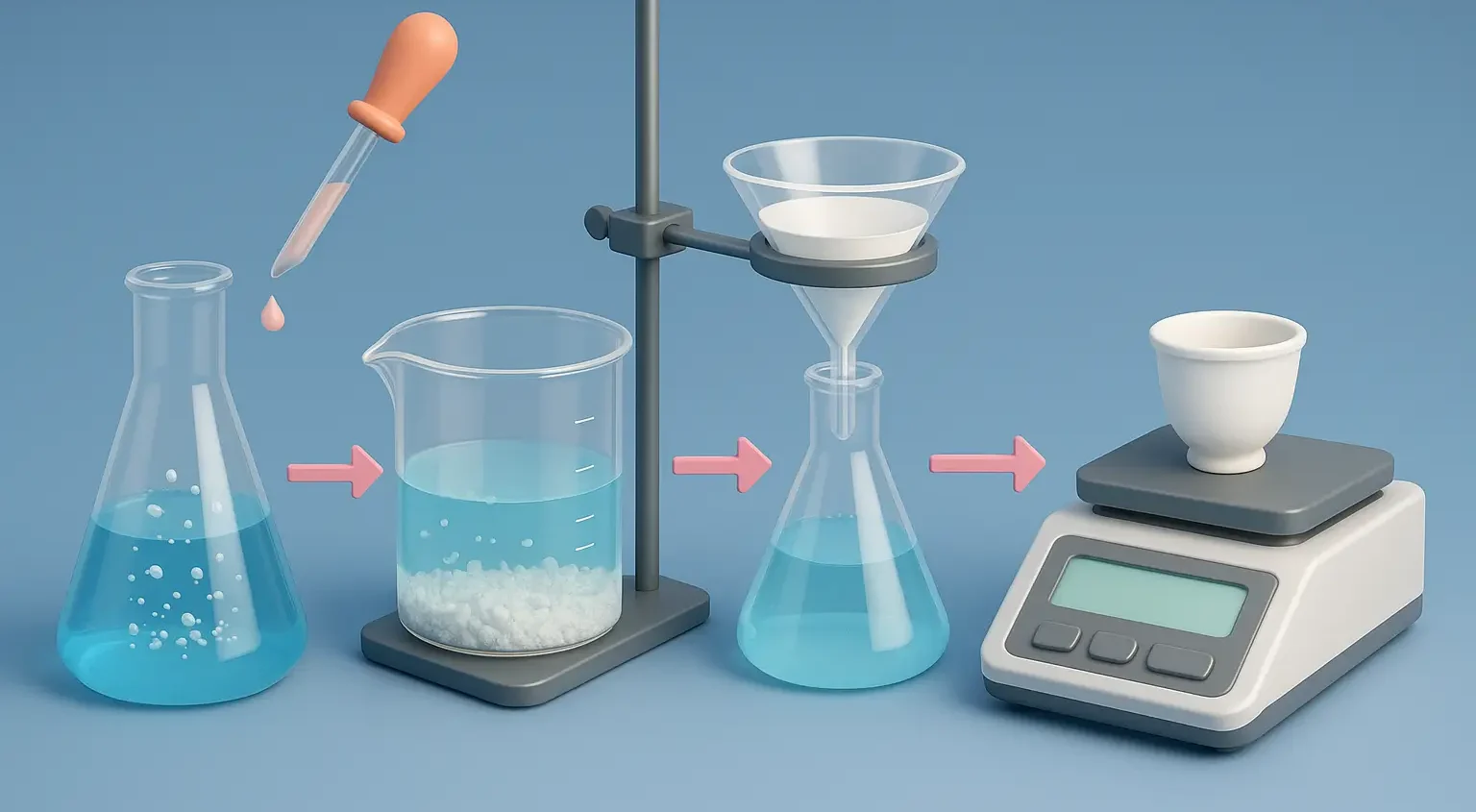
Gravimetric analysis of magnesium sulfate involves precipitating magnesium as magnesium ammonium phosphate and weighing the dried precipitate to determine magnesium content.
Principle:
- Gravimetric analysis is a quantitative method that determines the amount of an analyte based on its mass.
- The analyte is isolated, typically as a precipitate, which is then weighed to calculate the analyte’s concentration.
Steps in Gravimetric Analysis:
-
Sample Preparation:
- Weigh a known amount of the sample and dissolve it in a suitable solvent (e.g., distilled water).
- Filter to remove impurities if necessary.
-
Precipitation:
- Add a precipitating agent that reacts selectively with the analyte to form an insoluble precipitate.
-
Digestion:
- Allow the precipitate to settle, often by heating or standing, to improve purity and particle size.
-
Filtration:
- Filter the precipitate to separate it from the solution using appropriate filter paper.
-
Washing and Drying:
- Wash the precipitate to remove impurities, then dry it in an oven or desiccator to remove moisture.
-
Weighing:
- Weigh the dried precipitate using an analytical balance.
Calculation:
- Use the mass of the precipitate and the stoichiometry of the reaction to determine the analyte’s concentration.
- This concise process ensures the accurate determination of an analyte based on its mass.
Advertisements