- Hückel’s rule is a criterion used to determine if a molecule is aromatic.
- Aromatic compounds exhibit unusual stability and unique properties due to the delocalization of π electrons in a conjugated ring system.
Here are the key points:
- Cyclic Structure: The molecule must have a ring structure.
- Planarity: The molecule must be planar, which allows for effective overlap of p orbitals.
- Conjugated System: The molecule must have a conjugated system with alternating single and double bonds.
- π Electrons: The molecule must have a specific number of π electrons, calculated using the formula 4n+2, where n is a non-negative integer (0, 1, 2, …).
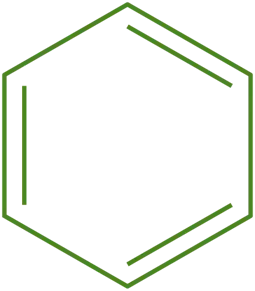
For example, benzene (C₆H₆):
- Cyclic Structure: Benzene has a ring structure.
- Planarity: Benzene is planar.
- Conjugated System: Benzene has alternating single and double bonds, forming a conjugated system.
- π Electrons: Benzene has 6 π electrons (one from each carbon).
-
Applying Hückel’s rule:
- 4n + 2 = 6
- Solving for n:
-
$n = \frac{6 – 2}{4} = 1$
-
-
- Since n = 1 is a non-negative integer, benzene satisfies Hückel’s rule and is aromatic.
- This explains benzene’s stability and unique properties.
-
Advertisements