- Kinetic properties of Colloids are related to the movement of colloidal particles due to thermal or external forces.
- Kinetic Properties of Colloids explain particle stability, transport, and dynamic interactions.
- These motions contribute to the stability and behavior of colloidal systems.

Advertisements
1. Brownian Motion

-
Definition:
- The random, zig-zag motion of colloidal particles due to continuous collision with molecules of the dispersion medium.
-
Discovered by:
- Robert Brown (1827)
-
Factors affecting:
- Particle size (smaller = more motion)
- Temperature (higher = faster motion)
- Viscosity of the medium
-
Significance:
- Prevents settling (adds to stability)
- Evidence of molecular motion
Advertisements
2. Diffusion
-
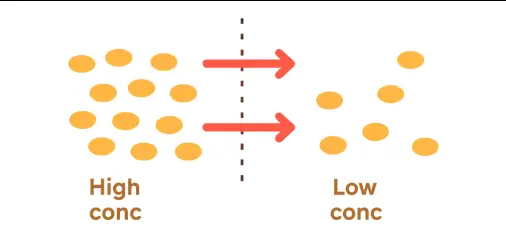
Definition:
- Spontaneous movement of colloidal particles from a region of higher concentration to lower concentration.
-
Note:
- Much slower than in true solutions
- Rate depends on particle size and medium viscosity
-
Use:
- Helps determine molecular weight of colloidal particles
- Affects uniform distribution of particles
Advertisements
3. Sedimentation
-
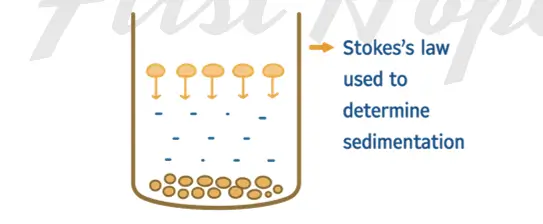
Definition:
- The settling of colloidal particles under the influence of gravity or centrifugal force.
- Normally very slow due to small size and Brownian motion
- Accelerated using centrifugation
-
Application:
- Separation and purification
- Determining particle mass or size via ultracentrifugation
4. Viscosity
-
Definition:
- The resistance to flow of a fluid, influenced by the presence of colloidal particles.
- Lyophilic sols increase viscosity significantly
- Lyophobic sols show minimal effect on viscosity
-
Applications:
- Estimating molecular size of polymers
- Industrial formulations (paints, cosmetics, food)
Advertisements