- Markovnikov’s rule is a fundamental principle in organic chemistry that helps predict the major product in electrophilic addition reactions involving unsymmetrical alkenes and polar reagents.
- This rule is crucial for determining the orientation of addition across the double bond in such reactions.
Principle of Markovnikov’s Rule
- When an unsymmetrical alkene reacts with a polar reagent (e.g., hydrogen halides like HX, where X can be Cl, Br, or I), Markovnikov’s rule states that:
- The hydrogen atom (H) from the reagent will add to the carbon atom of the alkene that already has the greater number of hydrogen atoms.
- Conversely, the halogen (X) or other part of the adding molecule will bond with the carbon atom that has fewer hydrogen atoms attached.
Rationale Behind Markovnikov’s Rule
- The underlying reason for orientation lies in the stability of the carbocation intermediates formed during the reaction process:
- A carbocation is more stable when it is attached to more alkyl groups due to the electron-donating effects of these groups, which can stabilize the positive charge through hyperconjugation and inductive effects.
- Therefore, the reaction pathway that leads to the formation of the more stable carbocation intermediate is favored, dictating the orientation of the addition.
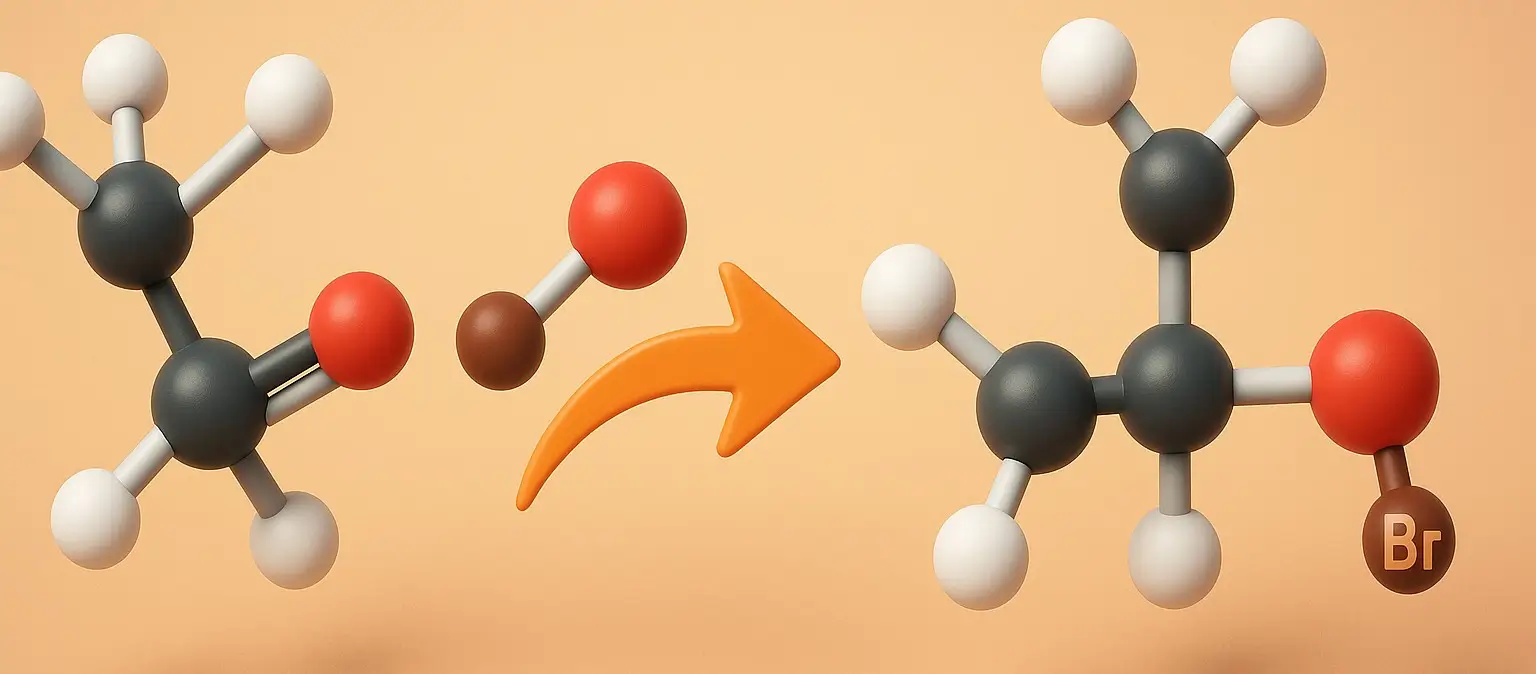
Example: Hydrohalogenation of Propene
- To illustrate This rule, consider the reaction of propene (CH₃CH=CH₂) with hydrogen bromide (HBr):
-
Reaction:
- CH₃CH=CH₂ + HBr → CH₃CHBrCH₃ (major product) or CH₃CH₂CH₂Br (minor product)
-
Outcome:
- The hydrogen atom from HBr preferentially adds to the middle carbon of propene, which already has two hydrogen atoms, while the bromine atom adds to the end carbon, which has only one hydrogen atom.
- This results in the formation of 2-bromopropane (CH₃CHBrCH₃) as the major product, following the above rule.
-
Application and Limitations of Markovnikov’s Rule
-
Application:
- Markovnikov’s rule is primarily applied to electrophilic addition reactions of alkenes, aiding in accurately predicting the outcome of such reactions.
-
Limitations:
- Markovnikov’s rule is best suited for predicting the outcomes of electrophilic addition reactions.
- Its applicability may not extend accurately to other reaction types involving alkenes, especially in the presence of peroxides or under specific conditions that favor anti-Markovnikov orientation.
Click Here to Watch the Best Pharma Videos