- Measurement of Surface can be measured using various techniques, each based on different physical principles.
- Below are the commonly used methods:
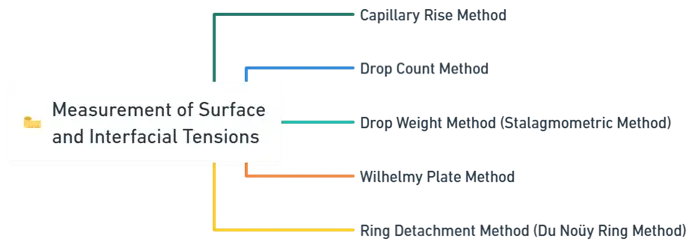
1. Capillary Rise Method for Measurement of Surface and Interfacial Tensions
-
Principle:
- This method relies on the height a liquid rises in a capillary tube due to surface tension.
- The liquid rises because of adhesive forces between the liquid and the capillary walls, balanced by gravitational force.
-
Formula:
-
-
-
-
$\gamma = \frac{h r \rho g}{2 \cos \theta}$
-
-
-
- where:
- γ = surface tension (N/m)
- h = height of liquid rise (m)
- r = radius of capillary tube (m)
- ρ = liquid density (kg/m³)
- g = gravitational acceleration (m/s²)
- θ = contact angle
-
-
Advantages:
- Simple and inexpensive
- Suitable for clean liquids
-
Limitations:
- Contact angle (θ) can be difficult to measure
- Not suitable for highly viscous or contaminated liquids
2. Drop Count Method for Measurement of Surface and Interfacial Tensions
-
Principle:
- Measures surface tension by counting the number of drops formed from a specific volume of liquid.
- A liquid with higher surface tension forms fewer but larger drops.
-
Formula:
-
-
-
-
$\gamma \propto \frac{V}{N}$
-
-
-
- where:
- γ = surface tension
- V = volume of liquid (mL)
- N = number of drops
-
-
Advantages:
- Simple and requires minimal equipment
-
Limitations:
- Drop formation is influenced by external factors like temperature and humidity
- Less accurate than other methods
3. Drop Weight Method (Stalagmometric Method)
-
Principle:
- Based on the weight of a drop detaching from a capillary tube.
- The surface tension balances the gravitational force until the drop detaches.
-
Formula (for a perfect sphere assumption):
-
-
-
$\gamma = \frac{mg}{2\pi r}$
-
-
-
- where:
- m = mass of the drop (kg)
- g = gravitational acceleration (m/s²)
- r = radius of the capillary (m)
-
Advantages:
- More precise than the drop count method
- Useful for studying interfacial tension
-
Limitations:
- Correction factors are needed due to non-spherical drop shapes
- Requires accurate mass measurement
4. Wilhelmy Plate Method
-
Principle:
- A thin plate (often platinum) is suspended and partially submerged in a liquid.
- The force exerted by the liquid’s surface tension on the plate is measured.
-
Formula:
-
-
-
-
$\gamma = \frac{F}{l}$
-
-
-
- where:
- γ = surface tension (N/m)
- F = force due to surface tension (N)
- l = perimeter of the plate in contact with the liquid (m)
-
-
Advantages:
- Highly accurate and suitable for dynamic measurements
- Can measure both surface and interfacial tensions
-
Limitations:
- Requires precision in force measurement
- Sensitive to contaminants
5. Ring Detachment Method (Du Noüy Ring Method)
-
Principle:
- A platinum ring is placed on a liquid surface and slowly pulled upward.
- The maximum force required to detach the ring from the surface is measured.
-
Formula:
-
-
-
-
$\gamma = \frac{F_{\text{max}}}{4\pi R}$
-
-
- γ = surface tension (N/m)
- Fmax = maximum detachment force (N)
- R = radius of the ring (m)
-
-
-
Advantages:
- Suitable for both surface and interfacial tension
- Provides consistent results
-
Limitations:
- Requires calibration and correction factors
- Not suitable for highly viscous liquids