Mercaptopurine is an anti-neoplastic antimetabolite used to treat leukemia by interfering with DNA and RNA synthesis in cancer cells.
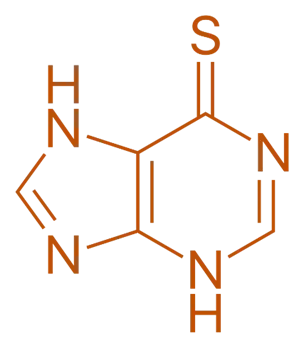
Structure of Mercaptopurine
- It is also known as 6-mercaptopurine (6-MP), is a purine analog with the following structural features:
- Purine Base: Similar to hypoxanthine.
- Thio Group: Contains a sulfur atom replacing the oxygen at position 6.
- Chemical Formula: C₅H₄N₄O S
Mode of Action
- Mercaptopurine functions as an antimetabolite and purine antagonist by:
- Incorporation into DNA and RNA: Disrupts nucleic acid synthesis and function.
- Inhibition of Purine Synthesis Enzymes: Blocks enzymes like amidophosphoribosyltransferase, reducing purine nucleotide synthesis.
- Induction of Apoptosis: Causes cytotoxicity in rapidly dividing cells, particularly in leukemic cells.
Advertisements
Uses of Mercaptopurine
- Acute Lymphoblastic Leukemia (ALL): As part of combination chemotherapy regimens.
- Chronic Myeloid Leukemia (CML): In certain treatment protocols.
- Inflammatory Bowel Disease (IBD): Such as Crohn’s disease and ulcerative colitis (off-label).
- Autoimmune Disorders: Including rheumatoid arthritis (off-label).
Structure-Activity Relationship (SAR)
- Purine Analog: The structural similarity to purine bases allows incorporation into DNA/RNA, disrupting nucleic acid function.
- Thio Group at Position 6: Enhances binding to target enzymes and incorporation into nucleic acids, increasing cytotoxicity.
- Substitution Patterns: Modifications on the purine ring can affect binding affinity and selectivity for target enzymes.
Synthesis