Metal Hydride Reduction with NaBH₄ and LiAlH₄ converts carbonyls to alcohols, widely used in organic and medicinal chemistry.
Overview:
- Metal hydrides like sodium borohydride (NaBH₄) and lithium aluminium hydride (LiAlH₄) are used to reduce carbonyl compounds (aldehydes, ketones, esters, carboxylic acids, etc.) to alcohols.
Advertisements
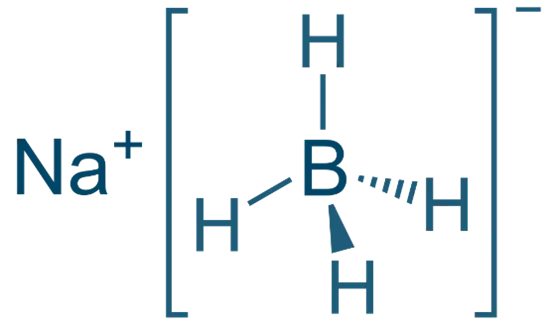
Sodium Borohydride (NaBH₄)
- Structure: Na⁺[BH₄]⁻ (a tetrahedral borohydride ion)

- Solubility & Stability: Stable in water and alcohol; can be used in aqueous or alcoholic solutions.
Reactivity:
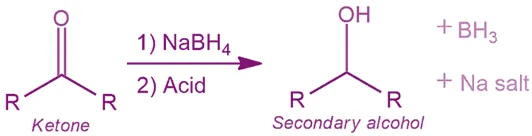
- Reduces: Aldehydes and ketones easily.
- Does not reduce: Esters, acids, amides (generally unreactive unless in harsh conditions).
Advertisements
Mechanism:
- The hydride ion (H⁻) from NaBH₄ attacks the electrophilic carbonyl carbon.
- This forms a tetrahedral alkoxide intermediate.
- Protonation of the alkoxide by solvent gives the alcohol.
Example:
- R-CHO (aldehyde) + NaBH₄ → R-CH₂OH (primary alcohol)
- R-CO-R’ (ketone) + NaBH₄ → R-CHOH-R’ (secondary alcohol)
Advertisements
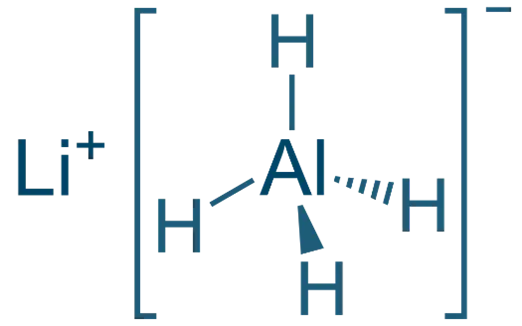
Lithium Aluminium Hydride (LiAlH₄)
- Structure: Li⁺[AlH₄]⁻

- Solubility: Reacts violently with water and alcohol — must be used in dry ethers like diethyl ether or THF.
Reactivity:
- Reduces: Aldehydes, ketones, esters, acids, amides, nitriles.
- Very strong reducing agent.
Advertisements
Mechanism (for ester reduction):
- LiAlH₄ donates a hydride to the carbonyl carbon.
- Tetrahedral intermediate collapses, ejecting the alkoxide leaving group.
- Another hydride adds to the aldehyde intermediate.
- Protonation gives a primary alcohol.
Example:
- R-COOH + LiAlH₄ → R-CH₂OH (primary alcohol)
- R-COOR’ + LiAlH₄ → R-CH₂OH + R’OH
- R-CONH₂ + LiAlH₄ → R-CH₂NH₂ (primary amine)
Click Here to Watch the Best Pharma Videos
Advertisements