Methods of Determination of Configuration of Geometrical Isomers involve techniques to identify cis-trans or E-Z arrangements in molecules.
Methods of Determination of Configuration of Geometrical Isomers
Geometrical isomerism (cis-trans or E-Z isomerism) occurs in compounds with restricted rotation (such as double bonds or cyclic structures), leading to different spatial arrangements of groups around the bond or ring.
There are three main methods to determine which isomer is which:
-
Physical Methods
These methods are based on measurable physical properties that differ between geometrical isomers:
-
-
Melting Point and Boiling Point
- Trans isomers are usually more symmetrical than cis isomers.
- Due to symmetry:
- Trans isomers pack better in the solid state → higher melting points.
- Cis isomers usually have higher boiling points because they often have net dipole moments (due to polar group alignment), leading to stronger intermolecular forces.
- Example:
- cis-but-2-ene: boiling point ~4°C
- trans-but-2-ene: boiling point ~1°C
-
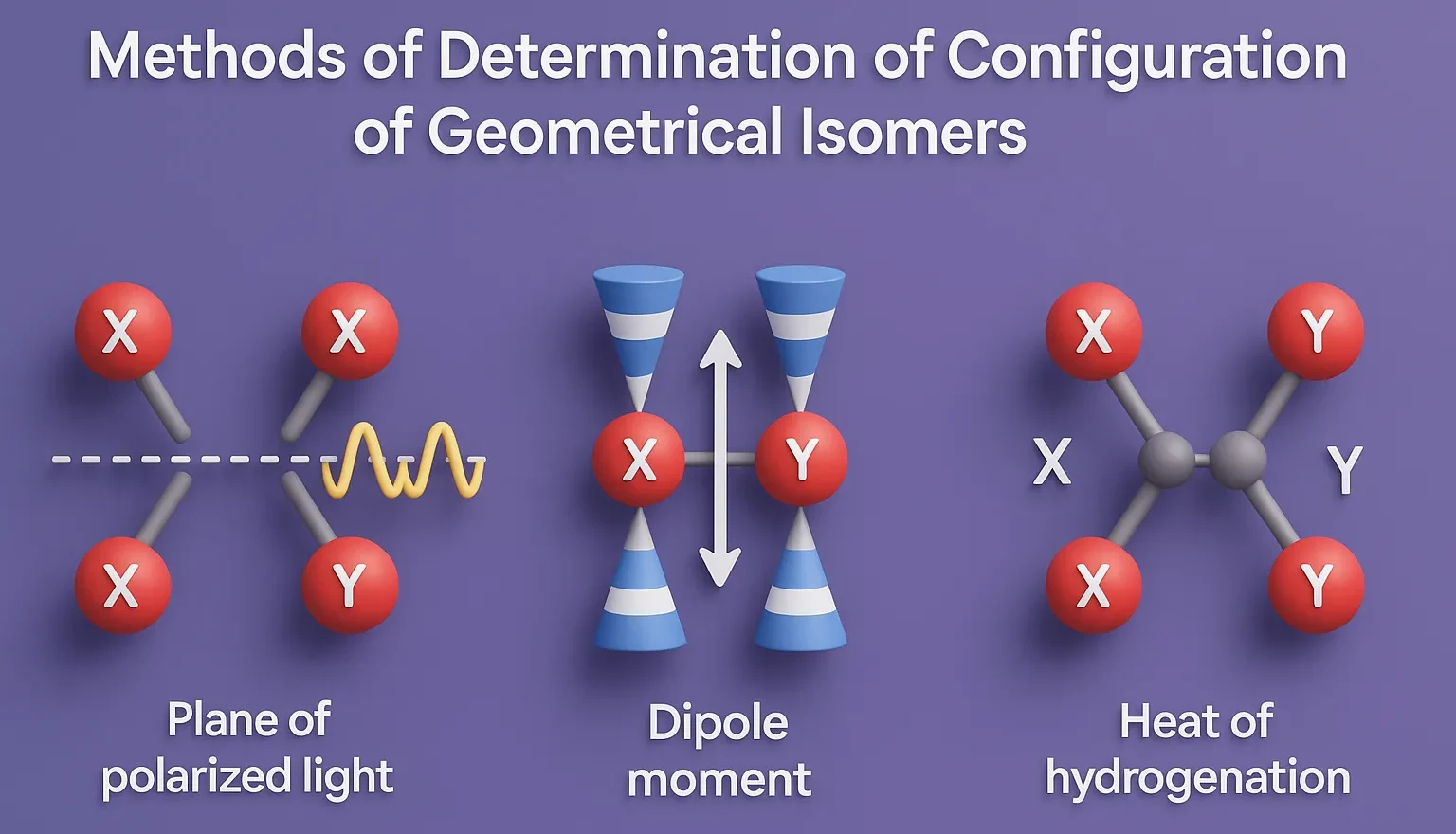
Dipole Moment
- Cis isomers often have higher dipole moments due to polar groups on the same side.
- Trans isomers often cancel out dipoles → lower net dipole.
- Measured via dielectric constant measurements or directly using specialized instrumentation.
-
Refractive Index and Density
- These may also differ slightly between isomers due to structural compactness and polarizability.
-
Spectroscopic Methods:
- UV-Vis Spectroscopy: Differences in conjugation can affect absorbance.
- IR Spectroscopy: The different group positions affect bond vibrations.
- NMR Spectroscopy: Coupling constants (³J) differ for cis and trans. Trans usually shows a higher coupling constant (~12-18 Hz) vs cis (~6-12 Hz).
-
-
Cyclisation Method
- This is a chemical method. It involves converting the geometrical isomers into cyclic compounds through intramolecular reactions.
-
Principle:
- If two functional groups are on the same side (cis), they may form a ring easily.
- If on opposite sides (trans), ring formation is difficult or impossible.
-
Example:
- Cis-butenedioic acid (maleic acid) forms a cyclic anhydride on heating (due to carboxyls being on the same side).
- Trans-butenedioic acid (fumaric acid) does not form the anhydride easily.
-
- So, ability to cyclize → suggests cis configuration.
- This is a chemical method. It involves converting the geometrical isomers into cyclic compounds through intramolecular reactions.
-
Chemical Conversion Method
- This involves converting one isomer into another or into a product whose structure (and hence configuration) is already known.
-
Interconversion
- Sometimes cis and trans forms can be interconverted via:
- Heat
- UV light (photochemical reactions)
- Catalysts
- By analyzing the product (e.g., by IR, melting point), we can deduce the original configuration.
- Sometimes cis and trans forms can be interconverted via:
-
Chemical Reaction Route
- A known stereospecific reaction can produce different products from cis/trans isomers.
- Example:
- Hydrogenation of cis-alkenes produces syn-addition
- Hydrogenation of trans-alkenes produces anti-addition
-
- This involves converting one isomer into another or into a product whose structure (and hence configuration) is already known.
If the hydrogenation product is known (cis or trans), then the configuration of the original alkene can be deduced.