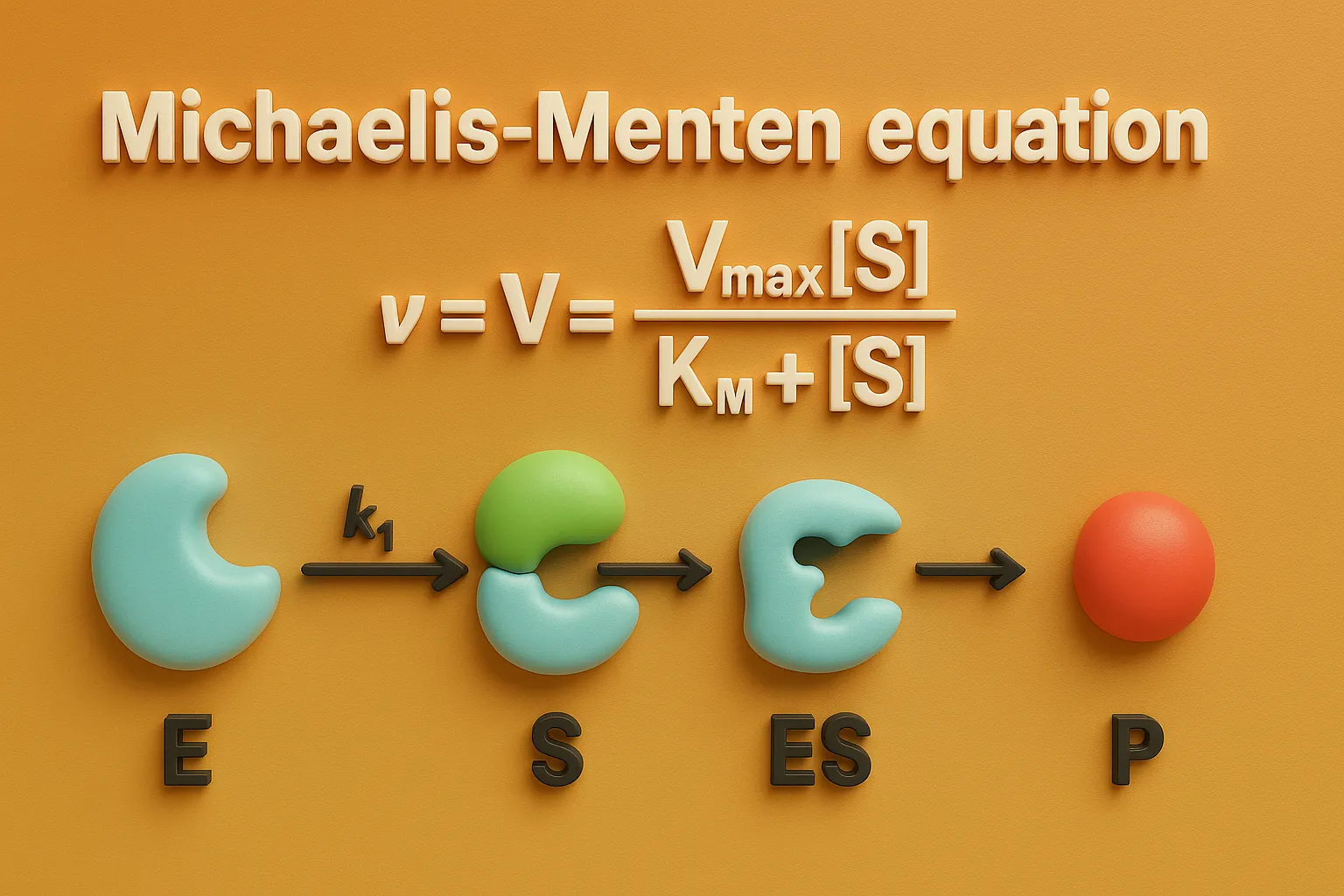Michaelis Menten equation describes enzyme kinetics by relating reaction rate to substrate concentration in pharmacology.
- The Michaelis-Menten equation describes the kinetics of enzyme-catalyzed reactions, first proposed by Leonor Michaelis and Maud Menten in 1913.
- It expresses the relationship between reaction rate (V) and substrate concentration ([S]):
$V = \frac{V_{\text{max}} \,[S]}{K_m + [S]}$
Where:
- V = reaction rate at a given substrate concentration
- Vmax = maximum reaction rate when the enzyme is fully saturated with the substrate
- [S] = substrate concentration
- Km = Michaelis constant, representing the substrate concentration at which the reaction rate is half of Vmax (i.e., Vmax/2), indicating enzyme affinity for the substrate
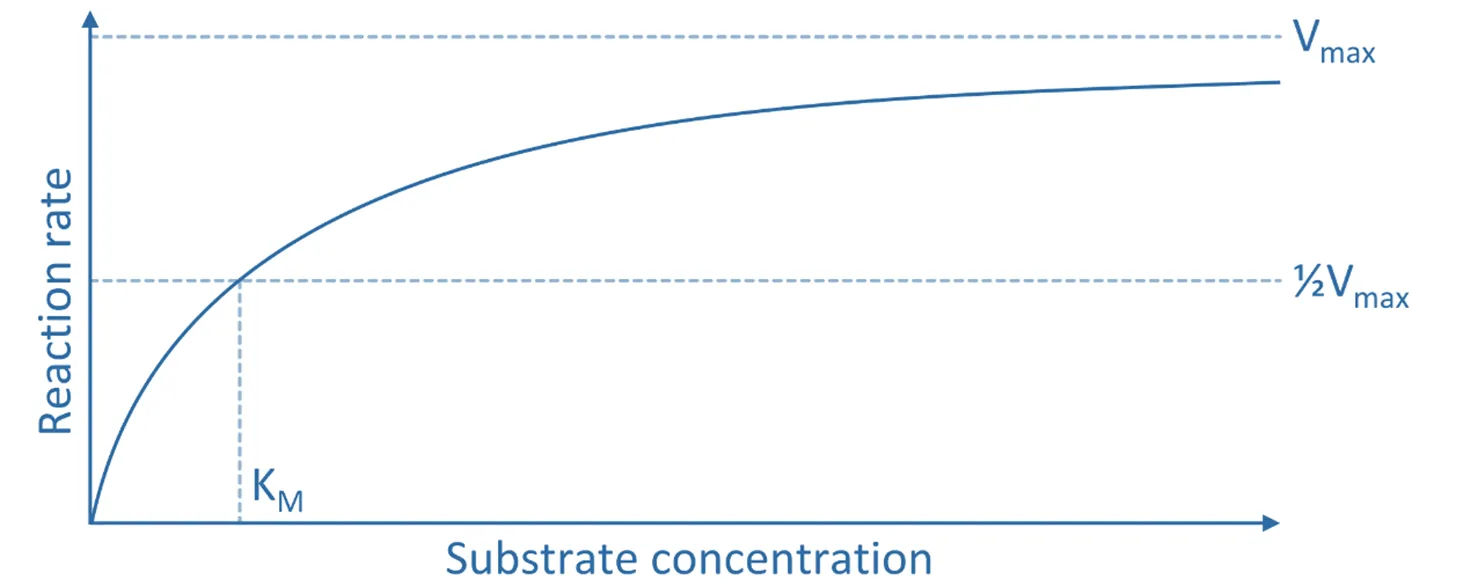
This equation assumes a steady-state condition for the enzyme-substrate complex and is useful in determining enzyme kinetics, such as affinity and catalytic efficiency.
Explanation with Examples of Drugs
-
Phenytoin:
- A classic example of a drug displaying nonlinear (dose-dependent) pharmacokinetics due to saturable hepatic enzyme metabolism.
- Even small increases in dose can cause disproportionately high increases in plasma levels and prolonged half-life.
-
Theophylline:
- At therapeutic doses, metabolism can become saturated, leading to unpredictable increases in plasma concentrations if dose adjustments are not carefully managed.
-
Ethanol:
- At moderate to high concentrations, the alcohol dehydrogenase pathway saturates, causing a constant elimination rate (i.e., zero-order kinetics over a certain range).
Click Here to Watch the Best Pharma Videos