- Neutralization curves are graphical representation of the pH of a solution during an acid-base titration.
- The pH is plotted on the y-axis, while the volume of the titrant (either acid or base) added is plotted on the x-axis.
- The shape of the neutralization curves depends on the nature of the acid and the base involved in the titration, such as strong acid-strong base, weak acid-strong base, strong acid-weak base, and weak acid-weak base titrations.
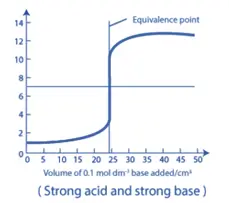
1. Strong Acid – Strong Base Titration:
- For a titration involving a strong acid and a strong base, the neutralization curve exhibits a rapid change in pH near the equivalence point.
- The pH at the equivalence point is close to 7.0, as the reaction between the strong acid and strong base produces water and a neutral salt.
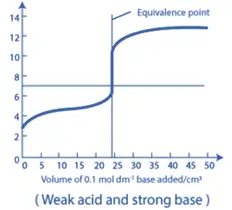
2. Weak Acid – Strong Base Titration:
- In a titration involving a weak acid and a strong base, the neutralization curve shows a slower increase in pH in the initial stages, followed by a rapid rise near the equivalence point.
- The pH at the equivalence point will be greater than 7.0, as the reaction produces a basic salt.
- A buffer region can be observed before the equivalence point, where the weak acid and its conjugate base can resist changes in pH.
Advertisements
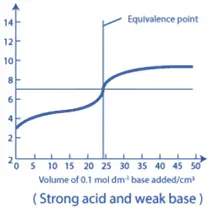
3. Strong Acid – Weak Base Titration:
- In this type of titration, the neutralization curve shows a rapid decrease in pH in the initial stages, followed by a slower decrease near the equivalence point.
- The pH at the equivalence point will be less than 7.0, as the reaction produces an acidic salt. Similar to the weak acid-strong base titration, a buffer region can be observed before the equivalence point, where the weak base and its conjugate acid can resist changes in pH.
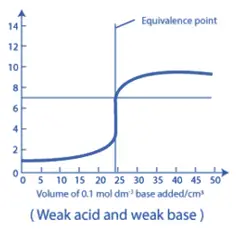
4. Weak Acid – Weak Base Titration:
- For a titration involving a weak acid and a weak base, the neutralization curve shows a gradual change in pH throughout the titration, with a less distinct inflection point at the equivalence point.
- The pH at the equivalence point can be close to, less than, or greater than 7.0, depending on the relative strengths of the acid and base.
- These titrations can be challenging due to the less distinct endpoints and the requirement for more precise pH measurement techniques.
Advertisements