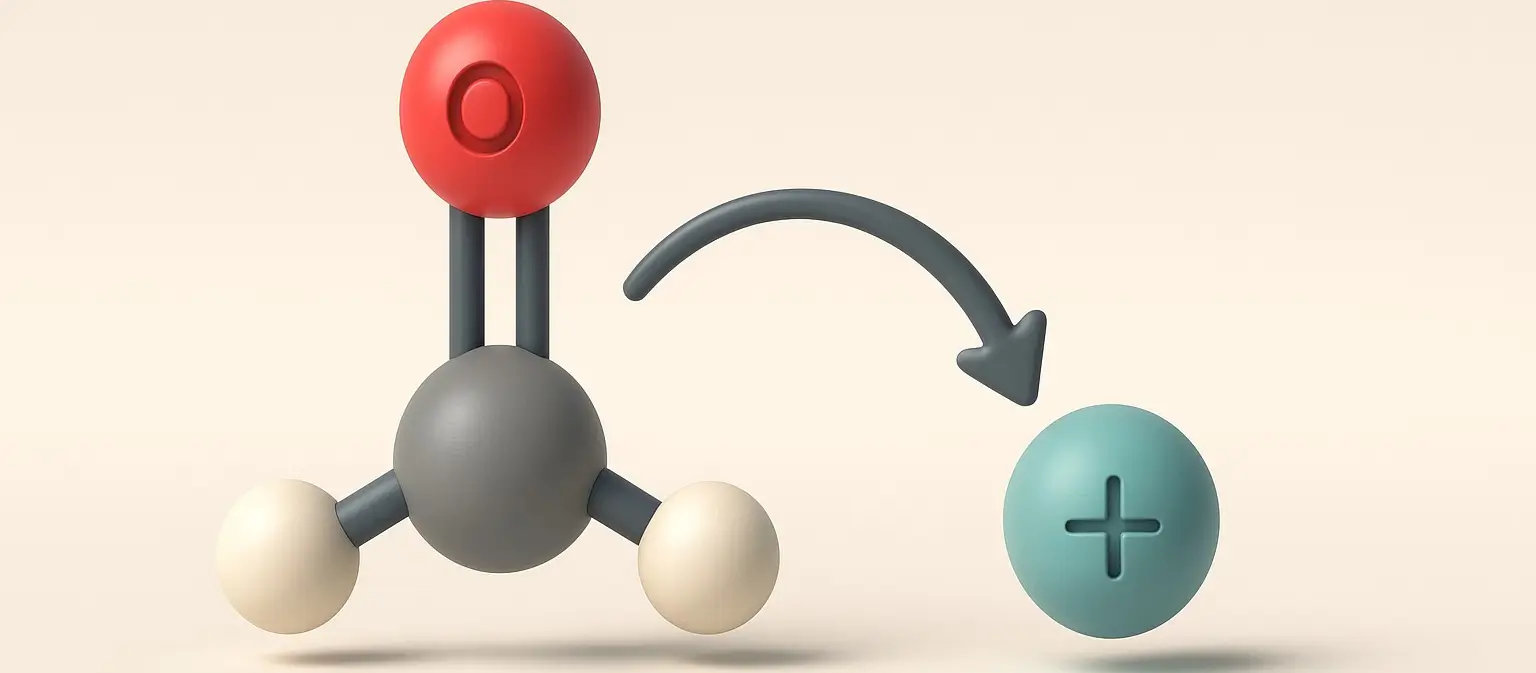
- Nucleophilic addition is a fundamental reaction mechanism in organic chemistry, particularly for the reactivity of carbonyl compounds like aldehydes and ketones.
- This process involves a nucleophile attacking an electrophilic center to form a new chemical bond.
- For carbonyl compounds, the electrophilic center is the carbon atom of the carbonyl group (C=O).
Mechanism of Nucleophilic Addition to Carbonyl Compounds
-
Nucleophilic Attack:
- The nucleophile, with a lone pair of electrons, attacks the electrophilic carbonyl carbon.
- The carbon is electrophilic due to the polarization of the C=O bond, where the oxygen atom pulls electron density away, making the carbon partially positive (δ+).
- Equation: Nu⁻ + R-CHO → Nu-R-CHO⁻
-
Formation of a Tetrahedral Intermediate:
- The attack breaks the π bond of the C=O group, pushing electrons onto the oxygen atom.
- This forms a tetrahedral intermediate with a negatively charged oxygen (O⁻).
- Intermediate Formation: Nu-R-CHO⁻
-
Proton Transfer:
- The negatively charged oxygen in the intermediate abstracts a proton (H⁺) from a proton source (solvent, acid, or conjugate acid of the nucleophile).
- This step neutralizes the charge on the oxygen, resulting in the formation of an alcohol.
- Protonation: Nu-R-CHO⁻ + H⁺ → Nu-R-CH(OH)
Example Reaction
- For a generic aldehyde (R−CHO):
- Step 1: The nucleophile (Nu⁻) attacks the carbonyl carbon, forming a tetrahedral intermediate.
- Step 2: The intermediate captures a proton (H⁺), yielding an alcohol (Nu−R−CH(OH)).
Click Here to Watch the Best Pharma Videos