This article explains about Oppenauer Oxidation oxidizes secondary alcohols to ketones using aluminium alkoxide and ketone acceptors.
Overview of Oppenauer Oxidation:
- Oppenauer oxidation is a mild, selective oxidation method used to convert secondary alcohols into ketones (and sometimes primary alcohols into aldehydes) under non-aqueous, basic conditions.
Advertisements
Reagents:
- Aluminum isopropoxide (Al(O-iPr)₃) – catalyst
- Excess ketone (commonly acetone) – hydrogen acceptor
- Non-aqueous solvent – typically benzene, toluene, or cyclohexane
- Mild heat
General Reaction:
R2CHOH + (CH3)2C=O —[Al(O-iPr)3] → R2C=O + (CH3)2CHOH
Advertisements
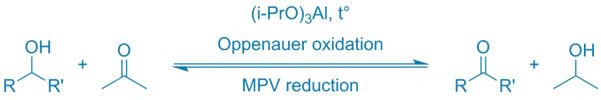
- The secondary alcohol is oxidized to a ketone.
- Acetone is reduced to isopropanol.
Advertisements
Mechanism:
Step 1: Formation of a coordination complex
- The hydroxyl group of the alcohol and the carbonyl oxygen of acetone both coordinate to Al(O-iPr)₃, forming a six-membered transition state.
Step 2: Hydride transfer
- A hydride (H⁻) is transferred from the alpha-carbon of the alcohol to the carbonyl carbon of acetone.
- The alcohol is oxidized to a ketone.
- Acetone is reduced to isopropanol.
Step 3: Product release
- The product ketone and isopropanol are released.
- The aluminum catalyst is regenerated.
Advertisements
Key Features of Oppenauer Oxidation:
- Mild and selective oxidation
- Ideal for acid-sensitive substrates
- Reverse of the Meerwein–Ponndorf–Verley reduction