Optical Isomerism is a type of stereoisomerism where compounds differ in how they rotate plane-polarized light due to chiral centers.
Definition of Optical Isomerism:
- Optical isomerism occurs when molecules can exist in two (or more) non-superimposable forms (stereoisomers) that differ in the way they interact with plane-polarized light.
- If a molecule can rotate the plane of polarized light, it is said to be “optically active.”
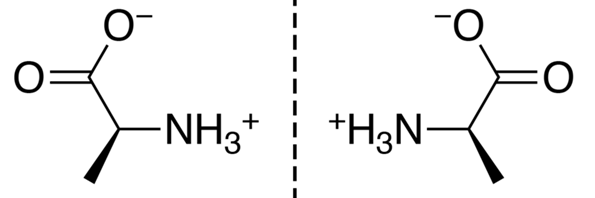
How Optical Activity is Measured
- Plane-Polarized Light: Ordinary light consists of waves vibrating in all planes. A polarizer (like a Nicol prism) filters out all but one plane of vibration, creating plane-polarized light.
- Polarimeter: An instrument used to measure how much a substance rotates plane-polarized light.
- If the substance rotates the plane to the right (clockwise), it is called “dextrorotatory” (labeled as “+” or “d-”).
- If it rotates the plane to the left (counterclockwise), it is called “levorotatory” (labeled as “−” or “l-”).
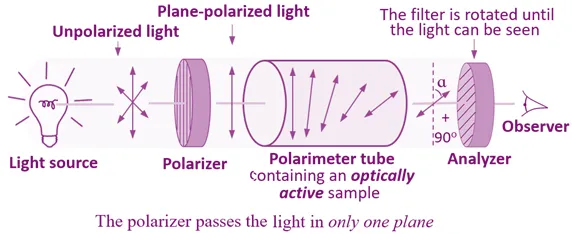
Types of Optical Isomers

-
Enantiomers
- Non-superimposable mirror images
- Identical physical properties (except for optical rotation)
- Rotate plane-polarized light equally but in opposite directions
- Represented as:
- (+)-enantiomer (dextrorotatory)
- (−)-enantiomer (levorotatory)
-
Diastereomers
- Stereoisomers that are not mirror images
- Occur when there are two or more chiral centers
- Physical and chemical properties are different
- Example: In a molecule with 2 chiral centers, (R,R) and (R,S) are diastereomers
-
Meso Compounds
- Contain chiral centers
- But are achiral overall due to an internal plane of symmetry
- Are optically inactive
- Superimposable on their mirror images
- Example: Meso-tartaric acid
Racemic Mixtures
- A 1:1 mixture of two enantiomers
- Optical activities cancel each other out
- The mixture is optically inactive
- Symbolized as: (±)-compound
Number of Optical Isomers
- If a compound has n chiral centers, the maximum number of stereoisomers is:
- 2ⁿ, where:
- Half are enantiomers
- The rest may be diastereomers
- Subtract meso forms (if any) to find optically active forms
Example of Optical Isomerism:
- A molecule with 2 chiral centers → up to 4 isomers
- (R,R), (S,S), (R,S), (S,R)
- If (R,S) and (S,R) are meso, then only 3 unique stereoisomers exist

