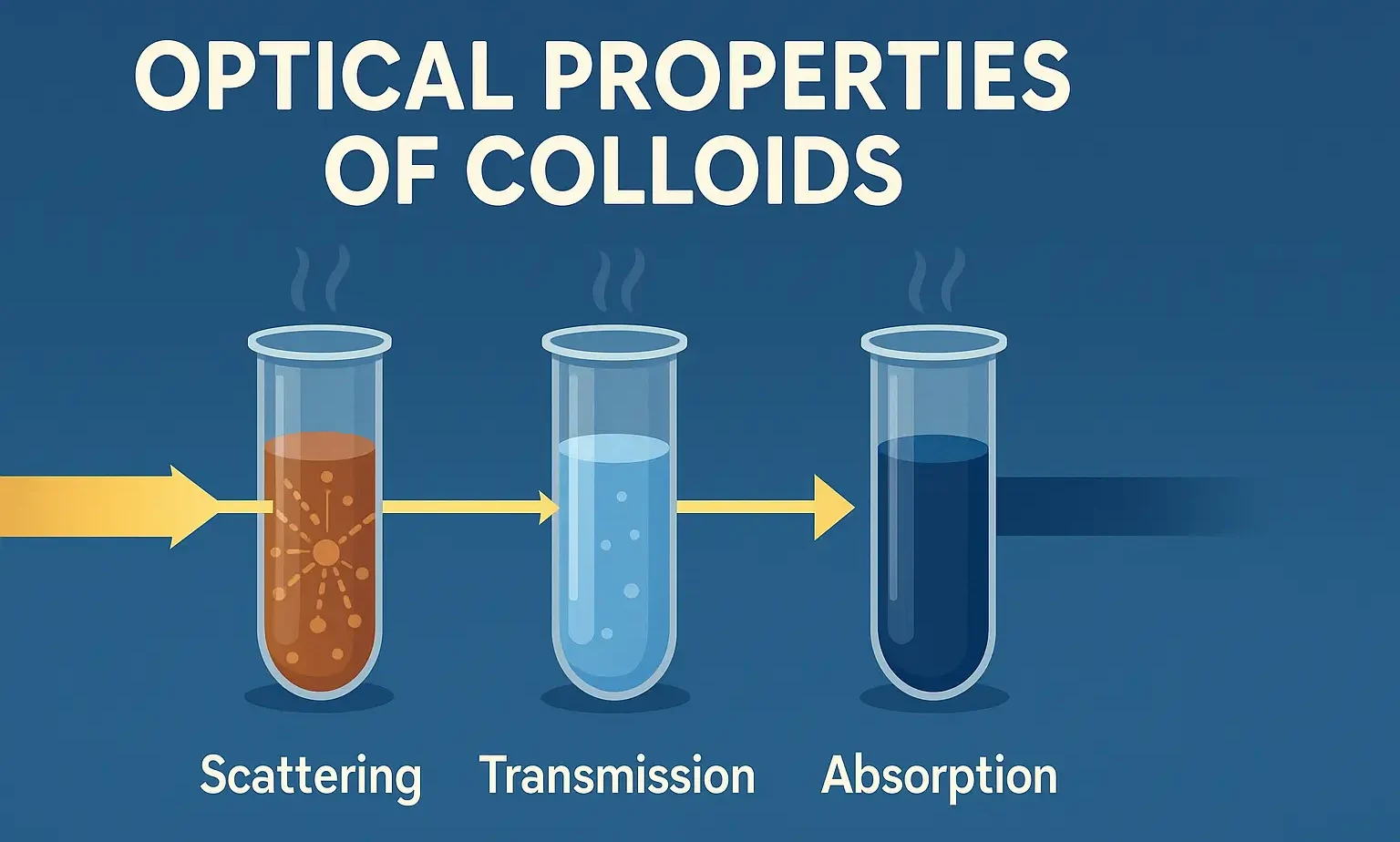
- Optical Properties of Colloids help in particle size analysis and stability studies of colloidal systems.
- Optical Properties of Colloids explain light scattering, Tyndall effect, and visibility of dispersed particles.
- Colloidal particles, due to their size (1–1000 nm), interact with light in unique ways. These optical effects help characterize and analyze colloidal systems.

Advertisements
-
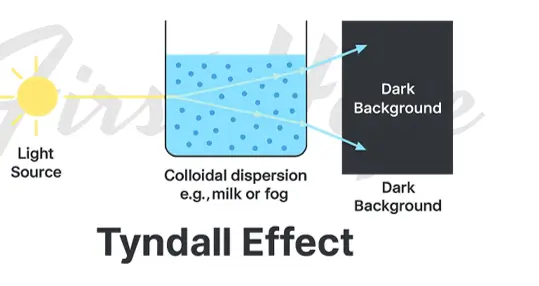
Tyndall Effect
Advertisements
-
Definition:
- The scattering of light by colloidal particles when a strong beam passes through a colloidal solution.
- Only observed in colloids, not in true solutions.
- The path of light becomes visible as a Tyndall cone.
- Caused by the difference in refractive indices between dispersed phase and medium.
-
Examples:
- Sunlight through mist or forest
- Projector beams in a dusty room
-
Significance:
- Helps distinguish between true solutions and colloids
- Basis for ultramicroscopy
-
Turbidity
Advertisements
-
Definition:
- The cloudiness or haziness of a colloidal solution due to light scattering by particles.
- Proportional to particle concentration and size
- Measured by turbidimeters or nephelometers
-
Application:
- Monitoring water purity
- Measuring colloid concentration in labs and industry
-
Ultramicroscopy
-
Definition:
- A method to observe colloidal particles individually as bright spots using scattered light.
-
Invented by:
- Siedentopf and Zsigmondy
-
Working:
- A strong side light is passed through the colloid.
- Particles scatter the light and appear as bright dots against a dark background.
-
Note:
- Only the scattered light is seen, not the actual particle.
- Does not reveal shape or structure, only presence.
Advertisements
-
Electron Microscopy
-
Definition:
- High-resolution microscopy using electron beams to directly visualize colloidal particles.
-
Advantages:
- Reveals size, shape, and structure
- Extremely high magnification and resolution
-
Used for:
- Nanotechnology
- Biological colloids (proteins, viruses)
- Studying catalysts, polymers
-
Ultrafilterability
Advertisements
-
Definition:
- Property of colloidal particles to pass through ordinary filter paper but not through ultrafilters.
- Ultrafilters: Special membranes (like collodion) with fine pores
- Used to separate colloidal particles from smaller molecules or ions
- Property of colloidal particles to pass through ordinary filter paper but not through ultrafilters.
-
Application:
- Purification of colloids (e.g., proteins)
- Determining particle size range