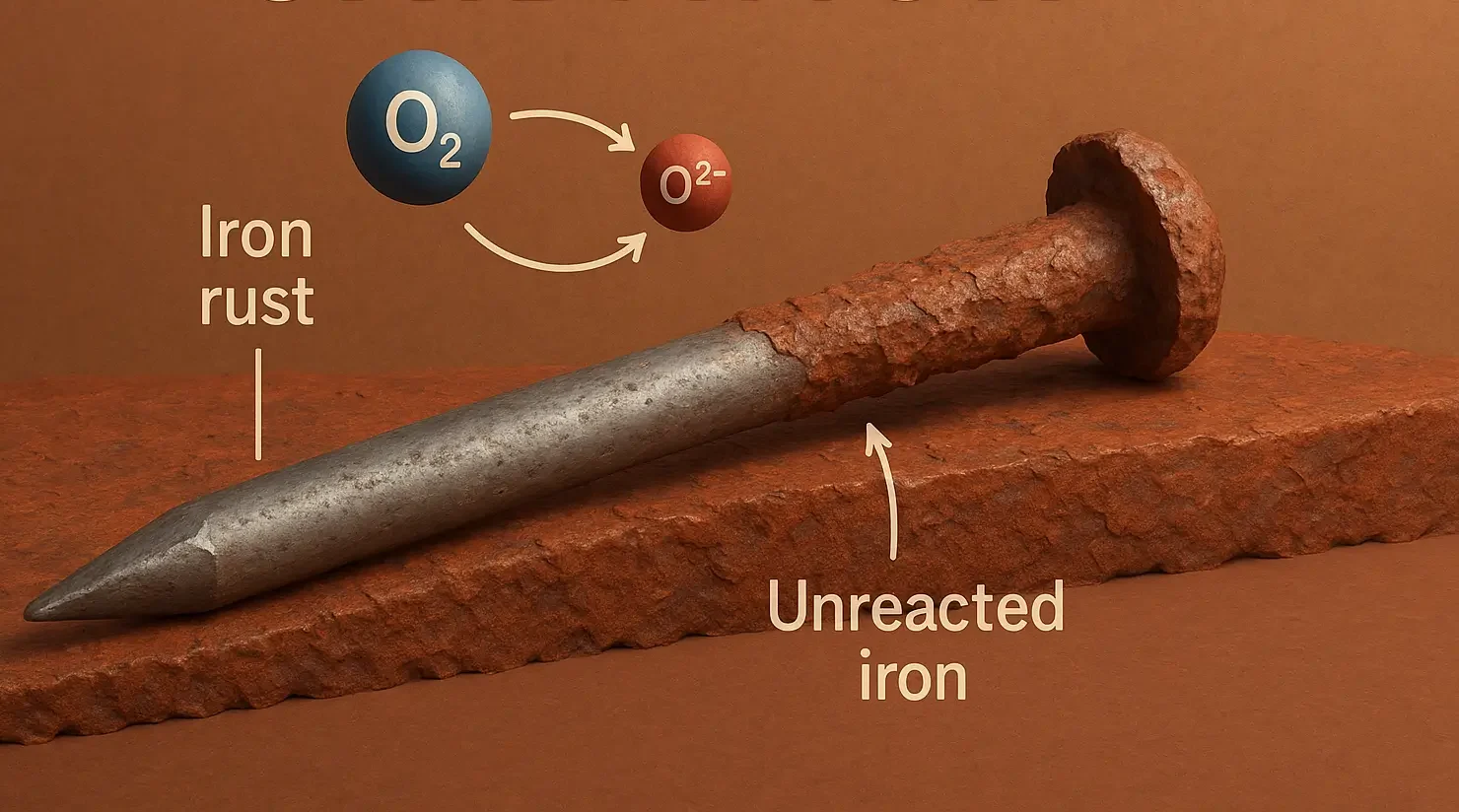
-
Definition:
- It is a chemical change involving electron loss, oxygen addition, or an increase in oxidation number during a reaction
- This process is essential in energy release, biological metabolism, corrosion, and many industrial applications.
-
Mechanism:
- Free radicals generated by heat, light, or metal ions initiate chain reactions, resulting in oxidative degradation.
Examples:
Example Reaction (Phenol Derivatives):
ArOH + (1/2) O₂ —[light]→ ArO* → ArO–OH
Example Reaction (Aldehydes to Carboxylic Acids):
RCHO + [O] → RCOOH
Prevention Strategies:
- Use of antioxidants (e.g., ascorbic acid, sodium metabisulfite).
- Packaging in oxygen-impermeable materials.
- Inert gases (e.g., nitrogen) in containers to displace oxygen.
- Chelating agents (e.g., EDTA) to remove metal ions that catalyze oxidation.
- Light-resistant packaging (amber glass bottles).