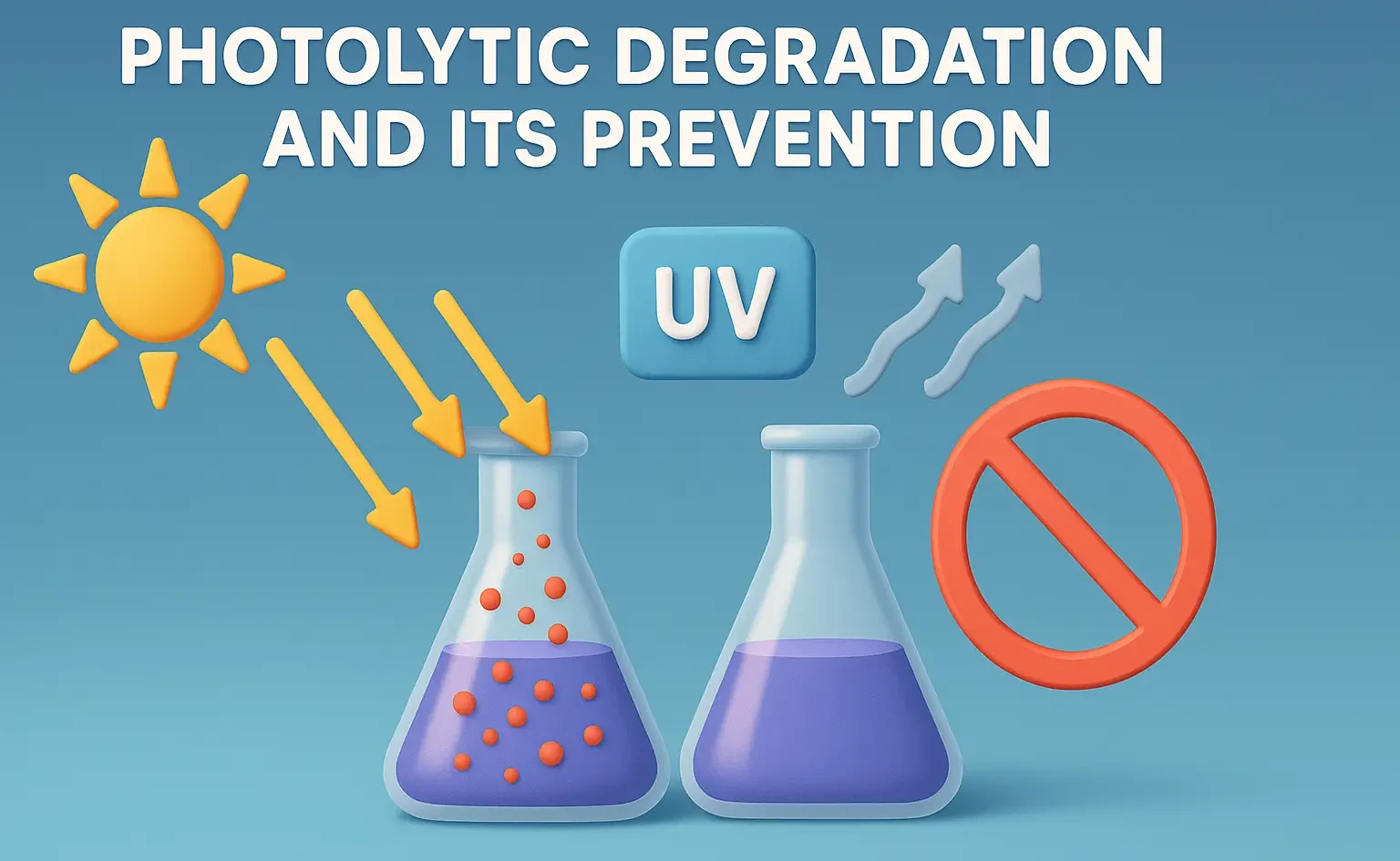
Definition:
- Photolytic (or photodegradation) is the degradation of drug substances due to exposure to light, especially UV and visible light.
- It leads to chemical breakdown and loss of potency, or formation of toxic by-products.
Mechanism:
- Light provides energy that excites electrons in drug molecules.
- Excited-state molecules may undergo bond cleavage, oxidation, or isomerization.
- Photodegradation can be:
- Direct: drug absorbs light directly.
- Indirect: light generates reactive species (e.g., singlet oxygen) that then degrade the drug.
Advertisements
Examples of Photolabile Drugs:
- Nifedipine (turns brown upon light exposure)
- Riboflavin
- Furosemide
- Phenothiazines
- Vitamin A and derivatives
Consequences:
- Potency loss
- Formation of colored or insoluble products
- Formation of toxic degradation products
- Regulatory non-compliance
Advertisements
Prevention Strategies:
-
Protective Packaging
- Amber glass bottles, opaque containers, or UV-blocking films are used.
- Aluminum foil blisters offer superior light protection.
-
Use of Light-Resistant Formulations
- Formulate with light stabilizers, UV absorbers, or antioxidants.
-
Coating of Tablets or Capsules
- Use film coatings that contain UV-protective agents.
-
Storage Recommendations
- Labeling: “Protect from light” or “Store in a dark place”.
- Store in lightproof boxes or cabinets.
-
Exclude Oxygen and Use Antioxidants
- Since photodegradation often involves oxidation, exclusion of oxygen and use of antioxidants can help.
-
Perform Photostability Testing (ICH Q1B Guidelines)
- Expose samples to defined light intensity (e.g., 1.2 million lux·hr visible + 200 watt·hr/m² UV).
- Evaluate degradation and appearance.
Click Here to Watch the Best Pharma Videos
Advertisements