Presentation of Molecules refers to different ways of representing molecular structures, such as Fischer, Newman, Sawhorse, and wedge-dash projections.
Presentation of Molecules
-
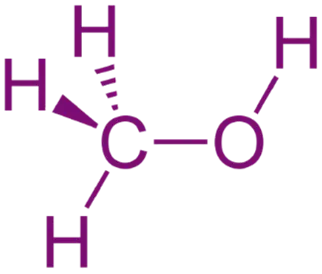
Wedge-Dash (3D) Projection
-
Purpose:
- Shows the actual 3D spatial orientation of atoms or groups.
-
Features:
- Solid wedge (▲): Bond coming out of the plane (towards you)
- Dashed wedge (▿ or dashed line): Bond going behind the plane
- Straight lines: Bonds in the plane of the paper/screen
-
Example:

-
-
Fischer Projection
-
Purpose:
- Common for sugars and amino acids
- Good for comparing D/L configurations
-
Rules:
- Vertical bonds: Go behind the plane
- Horizontal bonds: Come out toward the viewer

-
Steps to Convert Wedge-Dash to Fischer Projection
-
Identify the chiral center
- The central carbon is bonded to four different groups.
-
Orient the molecule correctly
- Make sure the horizontal bonds in the Fischer projection represent groups coming out of the plane (wedges).
- The vertical bonds represent groups going into the plane (dashes or behind the plane).
-
Rearrange the molecule
- Rotate the wedge-dash structure so that:
- The two groups on wedges (or wedge and regular line) are left and right (horizontal)
- The two groups on dashes/behind or straight lines are top and bottom (vertical)
- Rotate the wedge-dash structure so that:
-
Draw the Fischer projection
- Vertical line = bonds going into the plane (back)
- Horizontal line = bonds coming out of the plane (toward you)
- Place the correct groups based on their positions
Click Here to Watch the Best Pharma Videos
Advertisements