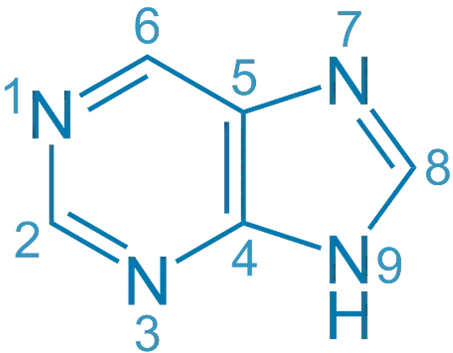
Purine is a bicyclic heterocyclic compound forming the basis of nucleic acids, coenzymes, and many pharmaceutical agents.
Structure
- A fused bicyclic ring: pyrimidine ring fused with imidazole.
- Molecular formula: C₅H₄N₄
- Found in DNA and RNA (adenine and guanine).

Advertisements
Synthesis
-
Traube Synthesis
- Multi-step process starting from 4,5-diaminopyrimidine, formic acid or formamide, then cyclization.
- Produces purine nucleus.
-
From Hypoxanthine or Xanthine
- Nitration or alkylation reactions on purine base.
- Used for making analogs (e.g., allopurinol).
-
Biosynthetic Pathway (Biological Relevance)
- In vivo, purines are synthesized via IMP (inosine monophosphate).
- Ribose-5-phosphate → PRPP → IMP → AMP/GMP
- In vivo, purines are synthesized via IMP (inosine monophosphate).
Medicinal Uses of Purine and Derivatives
- Nucleotides: Adenine and guanine are key DNA/RNA components.
- Anticancer Agents:
- 6-Mercaptopurine – inhibits DNA synthesis (used in leukemia).
- Gout Treatment:
- Allopurinol – xanthine oxidase inhibitor, lowers uric acid.
- Antivirals:
- Acyclovir – guanine analog, treats herpes simplex virus.
- Tenofovir – adenosine analog, used in HIV.
Advertisements
Advertisements