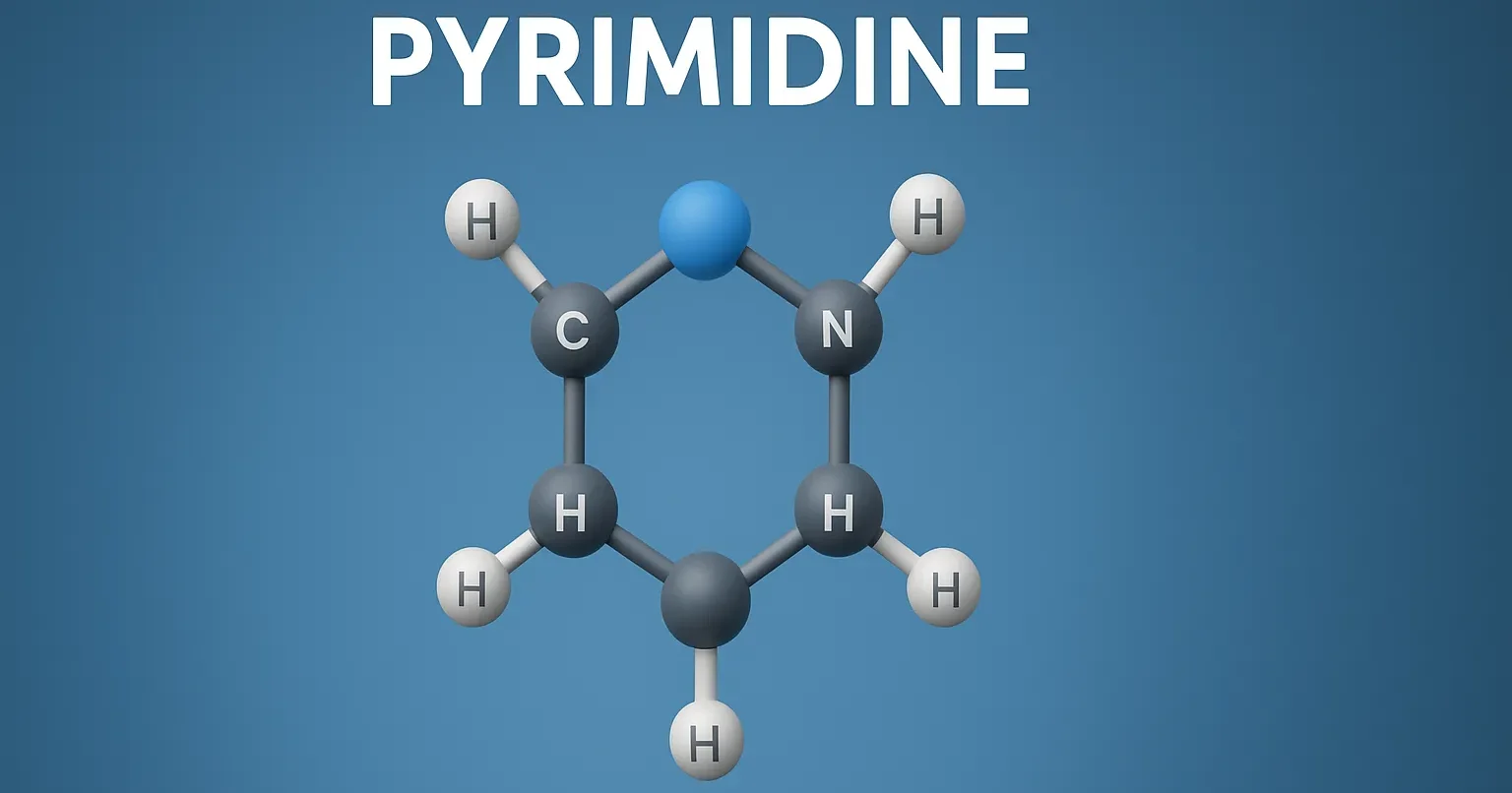
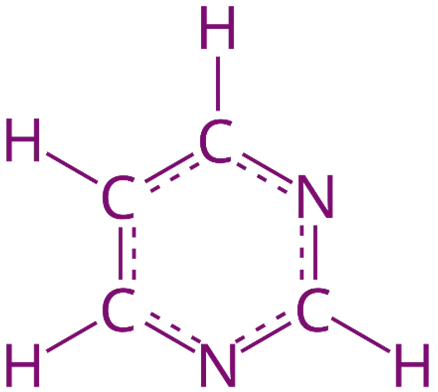
Pyrimidine is a six-membered heterocyclic compound with nitrogen atoms, essential in nucleic acids, drugs, and medicinal chemistry.
-
Structure
- It is a six-membered aromatic heterocycle with two nitrogen atoms at positions 1 and 3.
- Molecular formula: C₄H₄N₂

-
Synthesis
-
Biginelli Reaction (for substituted pyrimidines)
- Reactants: β-keto ester, aldehyde, and urea or thiourea.
- Conditions: Acidic catalysis, heating.
- Mechanism:
- Formation of iminium ion from aldehyde and urea.
- Nucleophilic attack by β-keto ester.
- Cyclization and dehydration form dihydropyrimidine, then oxidized to pyrimidine.
-
Pinner Synthesis
- From amidines and β-dicarbonyl compounds (like malonic esters).
-
From Barbituric Acid
- Barbituric acid is a pyrimidine derivative (malonylurea).
- Cyclization from urea and malonic acid derivatives.
-
-
Medicinal Uses of Pyrimidine and Derivatives
- Nucleic Acids: Cytosine, thymine, and uracil are natural pyrimidine bases.
- Antiviral Drugs:
- Zidovudine (AZT) – a thymidine analog used in HIV treatment.
- Lamivudine – cytidine analog for hepatitis B and HIV.
- Anticancer Agents:
- 5-Fluorouracil (5-FU) – uracil analog, inhibits thymidylate synthase.
- Antibacterial Agents:
- Trimethoprim-sulfamethoxazole – includes pyrimidine-like moiety.
Advertisements