R-S System of Nomenclature (Absolute Configuration) designates chiral centers as R or S based on substituent priority and spatial arrangement.
R-S System of Nomenclature (Absolute Configuration)
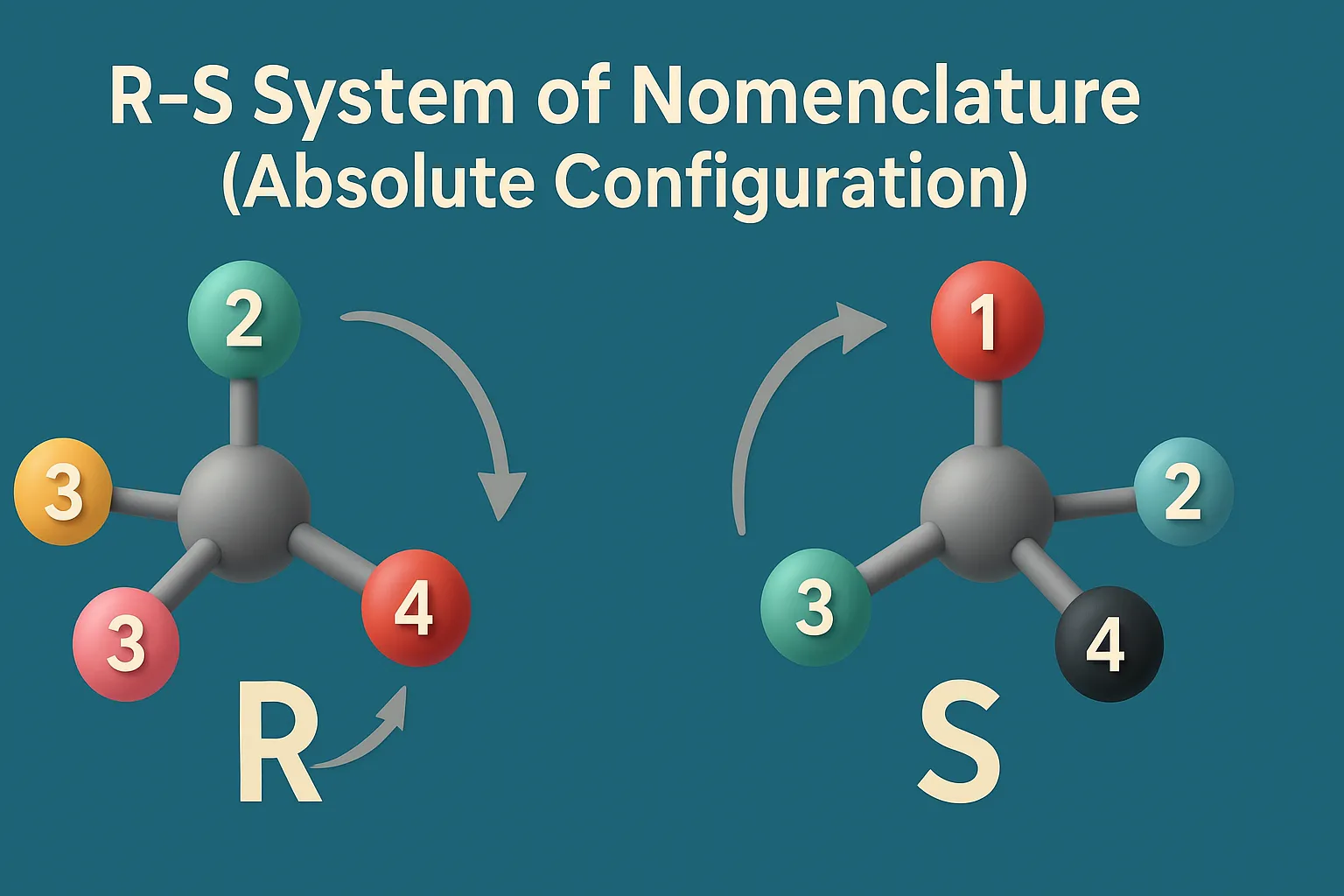
The R/S system gives the absolute configuration of a chiral center using the sequence rules.
Steps to assign R or S:
- Identify the chiral center.
- Assign priority (1–4) to the four groups attached to the chiral center using the sequence rules.
- Orient the molecule so that the lowest priority group (4) is pointing away from you (behind the plane).
- Observe the sequence from 1 → 2 → 3:
- If the sequence goes clockwise → the configuration is R (Rectus)
- If the sequence goes counterclockwise → the configuration is S (Sinister)
Example: 2-butanol (CH₃–CH(OH)–CH₂–CH₃)
- Chiral carbon = second carbon.
- Groups: OH, CH₃, CH₂CH₃, H
- OH > CH₂CH₃ > CH₃ > H
- Assign priorities.
- Make sure H is at the back.
- If sequence 1-2-3 is clockwise → R
- If counterclockwise → S
Advertisements
Note:
- Unlike the D-L system, the R/S system is universal, and can be applied to any type of compound (not just sugars or amino acids).
- R/S configuration does not correspond to D/L or +/–. They are different things:
- R/S = absolute configuration
- D/L = configuration based on glyceraldehyde
- +/– = optical rotation