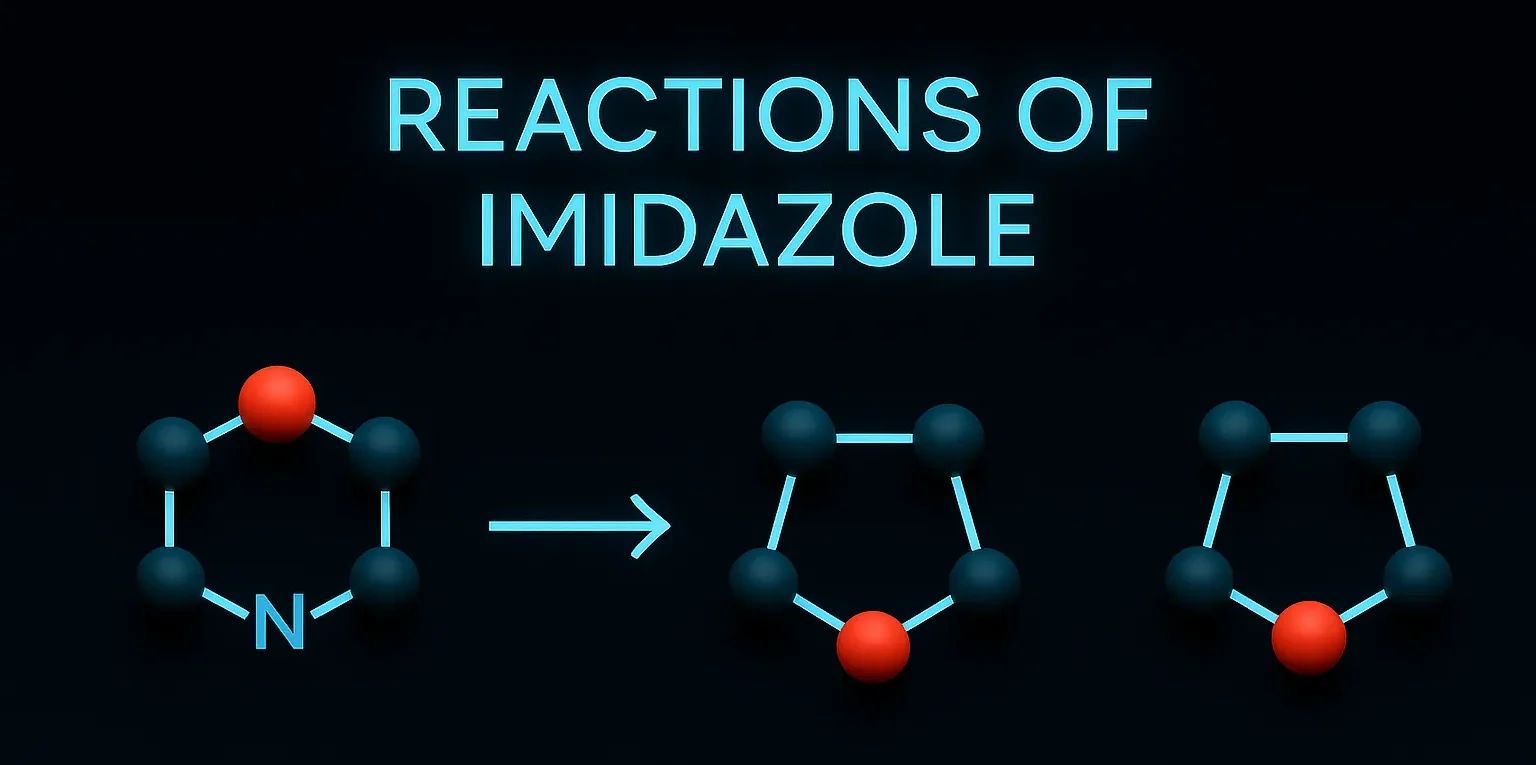
Reactions of Imidazole include electrophilic substitution at C-4/C-5 and nucleophilic substitution at C-2.
Reactions of Imidazole
-
Tautomerism:
- Prototropic tautomerism:
- 1H-imidazole ⇌ 3H-imidazole
- In practice, 1H-tautomer is more stable and dominates.
- Prototropic tautomerism:
-
Electrophilic Substitution Reactions (EAS):
- EAS occurs readily at position-4 or 5 due to high electron density.
- Nitration:
- Milder conditions than for benzene.
- Forms 4- or 5-nitroimidazole.
- Halogenation:
- Br₂ or Cl₂ → substitution at C-5 preferentially.
- Acylation/Friedel-Crafts-type Reactions:
- Possible at C-5.
- Requires mild Lewis acid or protected N-H group.
-
Nucleophilic Substitution Reactions (NAS):
- Occurs if EWG like NO₂ at C-4 or C-5.
- Rare in parent imidazole due to electron richness.
-
N-Alkylation and Acylation:
- N-1 can be alkylated easily:
- With alkyl halides → N-alkylimidazoles
- Acylation at N possible → amide-like derivatives.
-
Metal Coordination:
- Strong ligand via N-3.
- Coordinates with metals such as Cu²⁺, Fe³⁺.
- Histidine (in proteins) uses imidazole side chain to bind metal centers.
-
Ring Opening (Harsh Conditions):
- Possible under oxidative or nucleophilic attack with strong reagents.
- Rare and not synthetically common.
Advertisements