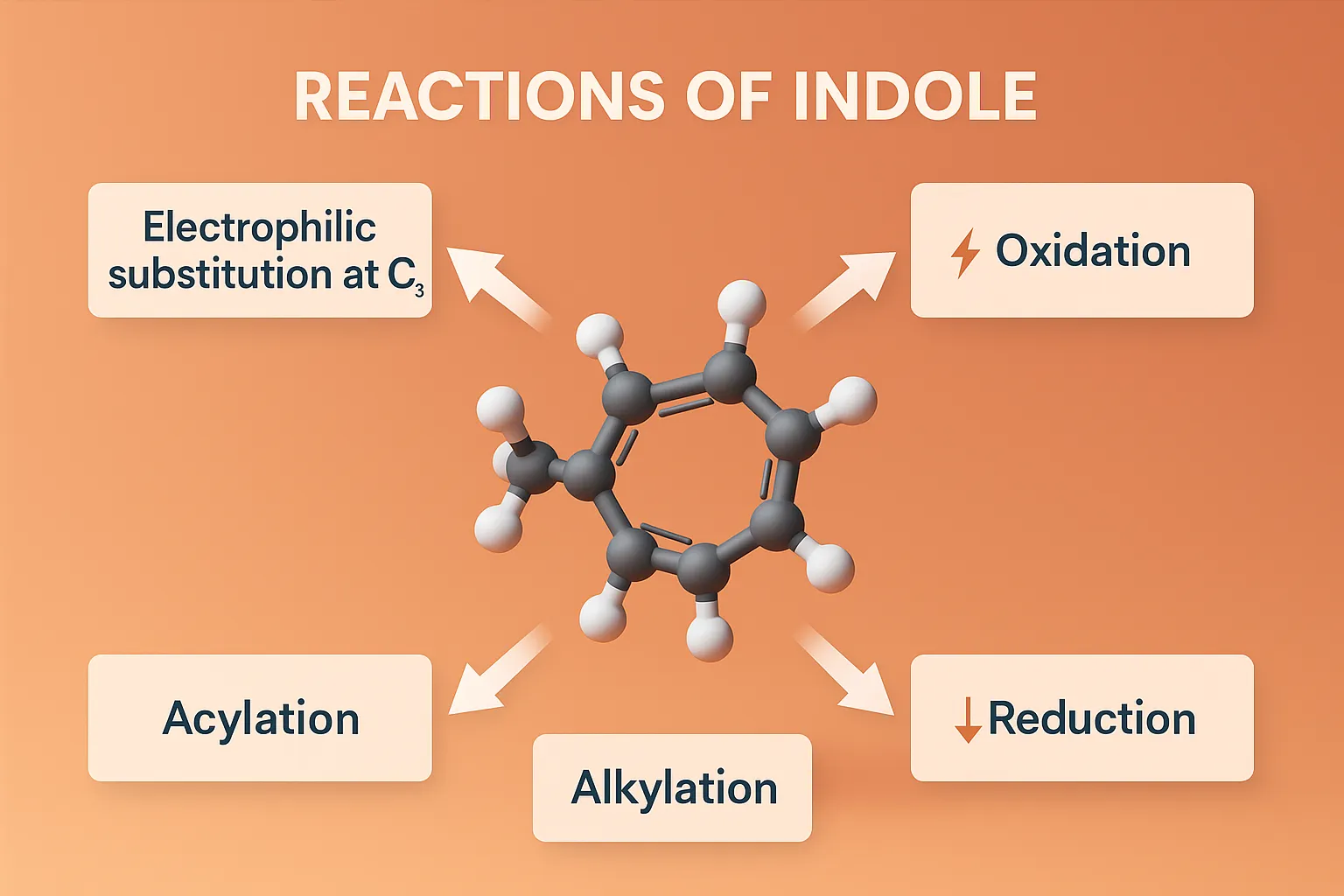
Reactions of Indole include electrophilic substitution, oxidation, reduction, and cyclization pathways vital in drug synthesis.
Reactions of Indole
-
Electrophilic Substitution (EAS)
- Very reactive at C-3 (due to resonance stabilization of carbocation intermediate).
- Secondarily reactive at C-2.
- Examples:
- Nitration: dilute HNO₃ → 3-nitroindole
- Halogenation: Br₂ → 3-bromoindole
- Friedel–Crafts acylation: at C-3
- Vilsmeier–Haack Reaction: POCl₃/DMF → 3-formylindole
-
N-Substitution
- Indole nitrogen can be acylated, alkylated to form:
- N-methylindole
- N-benzylindole
- These modulate reactivity and are important in drug design.
-
Oxidation
- Indoles can oxidize at C-2 or C-3:
- KMnO₄ → isatin (important building block)
- Oxygen + light → peroxides (decomposition)
- Indoles can oxidize at C-2 or C-3:
-
Reduction
- Mild hydrogenation → indoline (saturated pyrrole ring)
- Stronger → octahydroindole
-
Nucleophilic Attack
- Indole is less reactive to NAS but can undergo attack at electrophilic centers when substituted.