- SAR of Benzodiazepines (BZDs) highlights the importance of the 1,4-benzodiazepine nucleus for activity.
- SAR of Benzodiazepines (BZDs) shows how ring substitutions modify potency, duration, and receptor affinity.
- Benzodiazepines act primarily at the GABA-A receptor to enhance inhibitory neurotransmission.
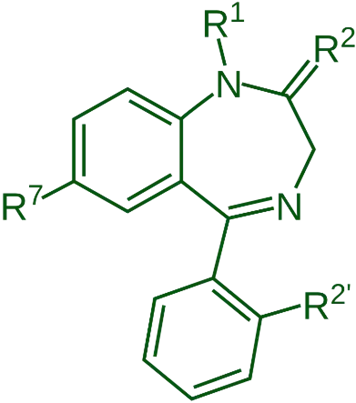
Key SAR of Benzodiazepines (BZDs) Points:
-
Aromatic Ring at Position 5 (C-5):
- A phenyl group at C-5 is important for activity.
- Substitutions at ortho-positions of this phenyl ring (like Cl or F) enhance activity.
- Para-substitution usually reduces activity.
-
Electron-Withdrawing Group at Position 7 (C-7):
- Halogens (Cl, NO₂) increase potency.
- Substitution at positions 6, 8, or 9 usually reduces activity.
-
Heterocyclic Ring Fusion at N-1 and C-2:
- The 1,4-diazepine ring is essential.
- Changes here can influence receptor binding.
-
Hydroxyl group at C-3:
- Enhances water solubility and metabolism (e.g., Lorazepam).
- Not always necessary but affects onset and duration.
-
N-1 Substitution:
- Alkyl groups (e.g., methyl in Diazepam) are tolerated.
- Bulky groups reduce activity.
-
Ring B Fusion (1,2-benzodiazepines):
- Some analogs (triazolo- or imidazo-rings) increase potency and duration (e.g., Alprazolam).
Click Here to Watch the Best Pharma Videos
Advertisements

