- SAR of Morphine Analogues highlights opioid receptor binding, guiding design of safer pain relievers.
- SAR of Morphine Analogues shows how structural changes modify analgesic potency and side effects.
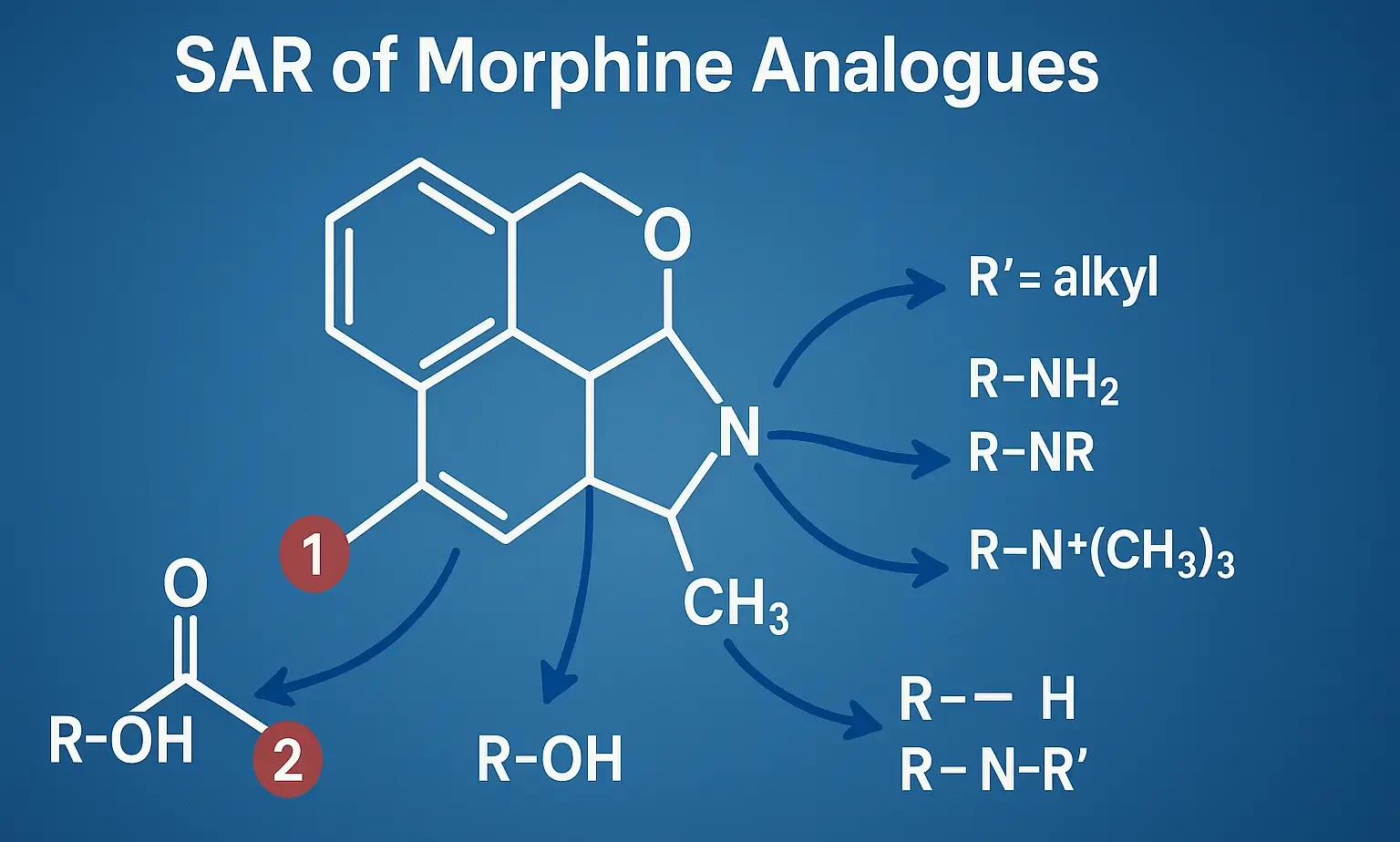
- Morphine has a rigid, pentacyclic ring system with functional groups critical to opioid activity.
Key Features of SAR of Morphine Analogues:
-
Phenolic OH at C-3:
- Essential for activity via H-bonding with opioid receptors.
- Methylation (e.g., codeine) reduces potency but improves oral bioavailability.
-
Alcoholic OH at C-6:
- Modifications affect potency/duration.
- Oxidation to ketone (e.g., oxymorphone) boosts potency.
- Acetylation (e.g., heroin) increases lipid solubility and brain entry.
-
C-7/C-8 Double Bond:
- Reduction (e.g., dihydromorphine) usually increases potency.
- Removal adds flexibility, possibly reducing selectivity.
-
Aromatic A-Ring:
- Needed for π–π interactions with receptors.
- Substitutions often reduce activity unless planarity/electron density are preserved.
-
Tertiary Amine at N-17:
- Critical for activity – binds receptor’s negative site.
- Small groups (methyl) = agonist.
- Bulkier groups (e.g., cyclopropylmethyl) = antagonist or mixed activity.
-
N-to-Aromatic Ring Distance:
- 2-carbon span is optimal.
- Altering these spacing lowers activity.
-
Rigid Ring Conformation:
- Fused ring rigidity maintains receptor-binding orientation.
- Flexibility (in some analogues) may affect potency/selectivity.
Click Here to Watch the Best Pharma Videos