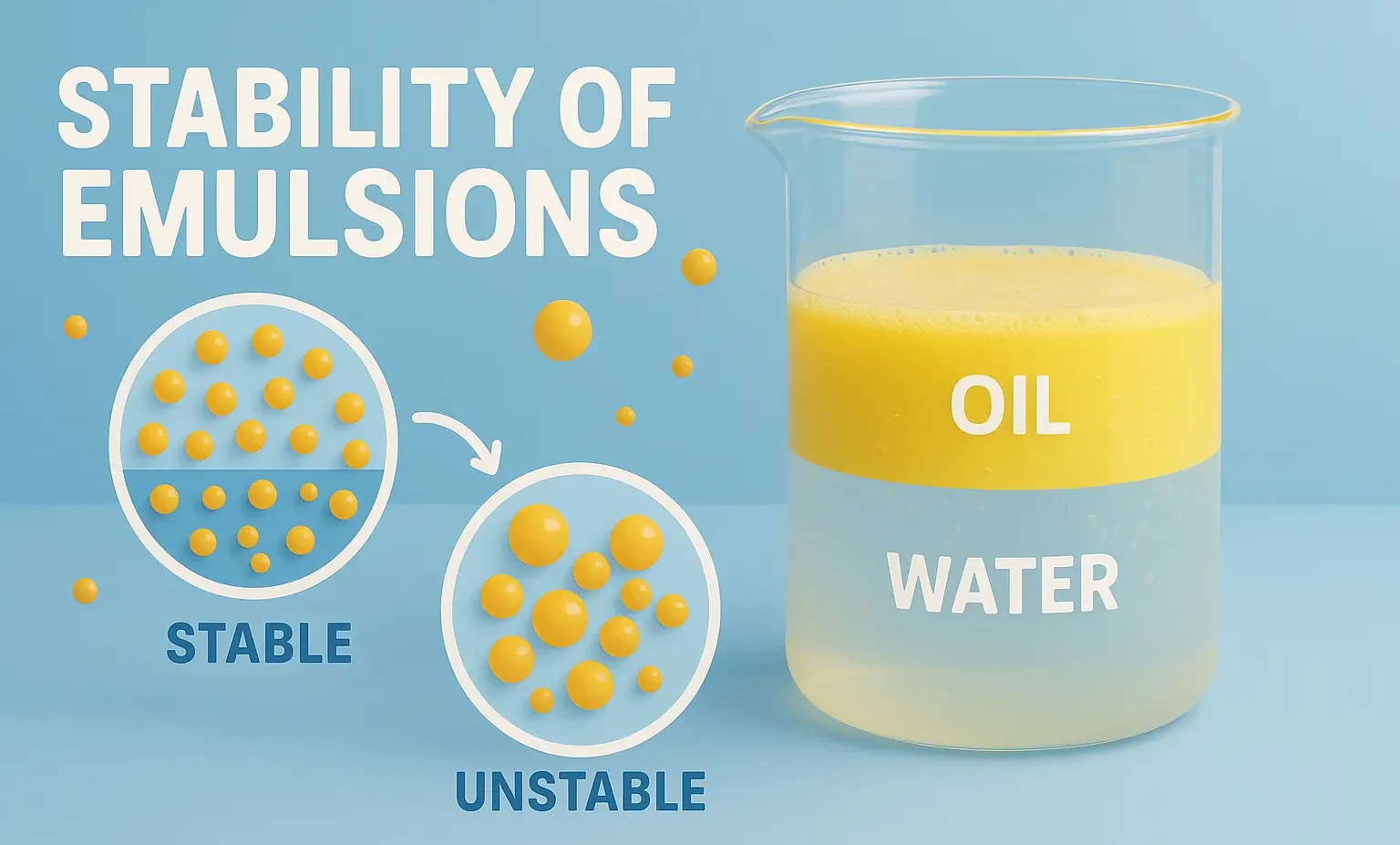
- Stability of Emulsions refers to the ability of dispersed droplets to resist coalescence and phase separation.
- It is vital in pharmaceuticals, foods, and cosmetics for product effectiveness.
- Stability refers to the ability of an emulsion to resist changes such as separation, creaming, cracking, or phase inversion over time.
Types of Instability in Emulsions
Instability may occur due to physical or chemical changes, including:
Advertisements
-
Creaming
- Upward or downward movement of dispersed droplets due to density difference.
- Reversible by shaking.
-
Coalescence
- Fusion of small droplets into larger ones.
- Can lead to complete separation.
-
Breaking (Cracking)
- Irreversible separation into two distinct layers (oil and water).
- Cannot be redispersed.
-
Flocculation
- Droplets aggregate but don’t merge.
- A precursor to coalescence.
-
Phase Inversion
- The internal and external phases switch (O/W ↔ W/O).
- Caused by temperature changes or excessive internal phase.
-
Changes in Physical and Chemical Properties
How to Improve Stability:
- Use appropriate emulsifiers (with suitable HLB value).
- Maintain optimum pH and temperature.
- Add viscosity enhancers (like gums).
- Store in cool, dark conditions to avoid oxidation or degradation.
Advertisements
Factors Influencing Emulsion Stability
- Droplet size: Smaller droplets = more stable
- Viscosity of continuous phase: Higher viscosity reduces movement and creaming
- Density difference between phases: Smaller difference = better stability
- Temperature: High temp accelerates instability
- Emulsifier: Proper choice and concentration is key
Click Here to Watch the Best Pharma Videos
Advertisements