Steady State Drug Levels describe equilibrium concentration reached with repeated dosing when drug input equals elimination.
Definition of Steady-State Drug Levels:
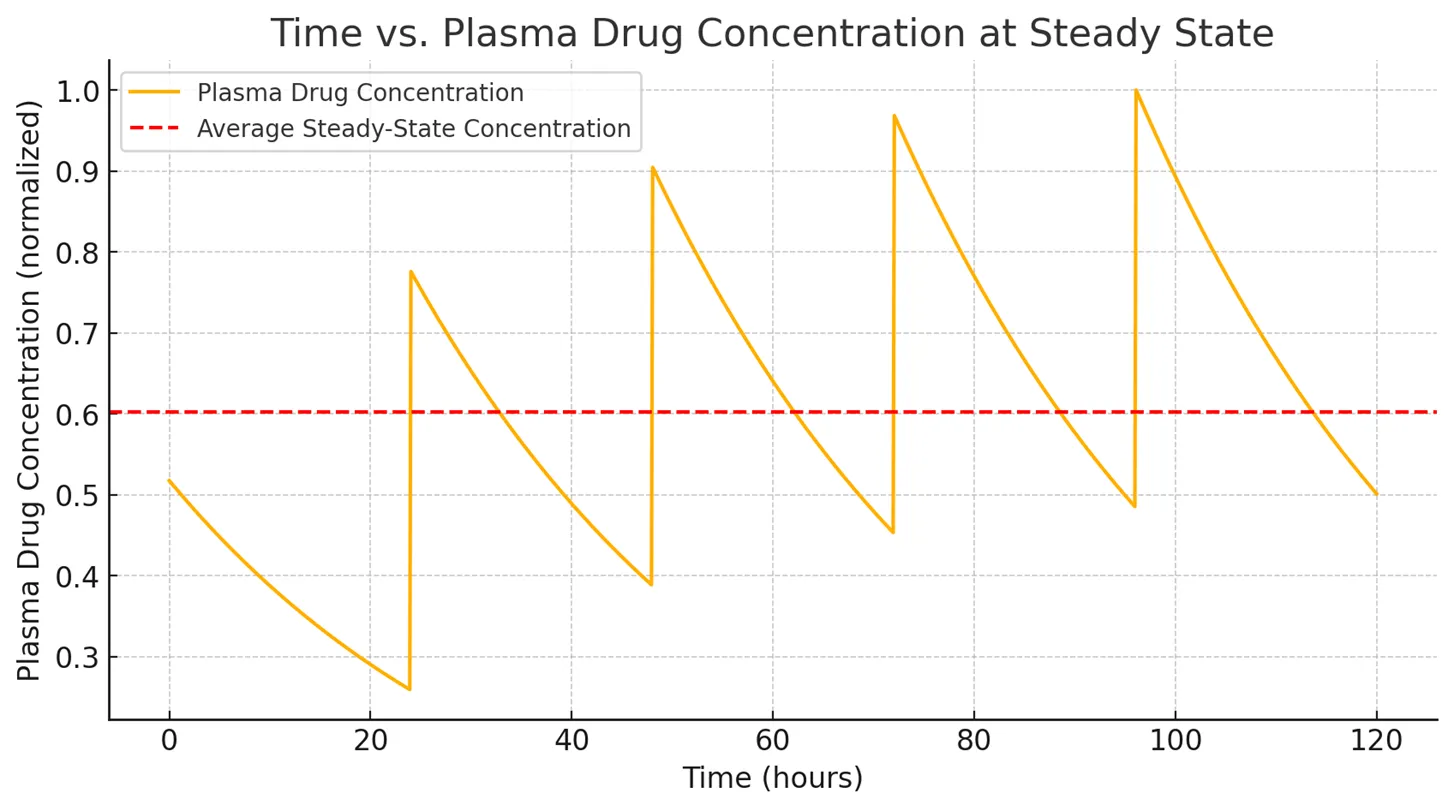
- Steady state (Css) is reached when the rate of drug administration = rate of drug elimination.
- Occurs after ~4-5 half-lives of continuous dosing.
Why It Matters
- Maintains consistent therapeutic levels
- Minimizes risk of toxicity or therapeutic failure
- Supports optimized dosing regimens
Key Parameters:
$C_{ss,\text{avg}} = \frac{F \cdot D}{Cl \cdot \tau}$
Advertisements
- F: bioavailability (1 for IV)
- D: dose
- Cl: clearance
- τ: dosing interval
Key Concepts
-
Time to Steady State:
- Typically reached after 4–5 half-lives with consistent dosing.
-
Graphical Representation:
- A plateau in drug concentration over time with small, regular fluctuations between doses.
Factors Affecting Steady-State Levels
-
Drug Half-Life (t½)
- Longer half-life = slower approach to steady state.
-
Dosing Interval (τ)
- Should align with the drug’s half-life to maintain levels within the therapeutic window.
-
Dose Size and Frequency
- Influence the average concentration at steady state.
-
Clearance and ADME
- Absorption, Distribution, Metabolism, Excretion, and drug clearance determine concentration profiles.
-
Patient-Specific Factors
- Age, weight, renal/hepatic function, genetics affect pharmacokinetics and time to steady state.
Clinical Relevance
- Understanding steady-state dynamics helps:
- Ensure efficacy
- Prevent toxicity
- Personalize treatment using pharmacokinetic models
Advertisements
