Stereoisomerism in Biphenyl Compounds (Atropisomerism)
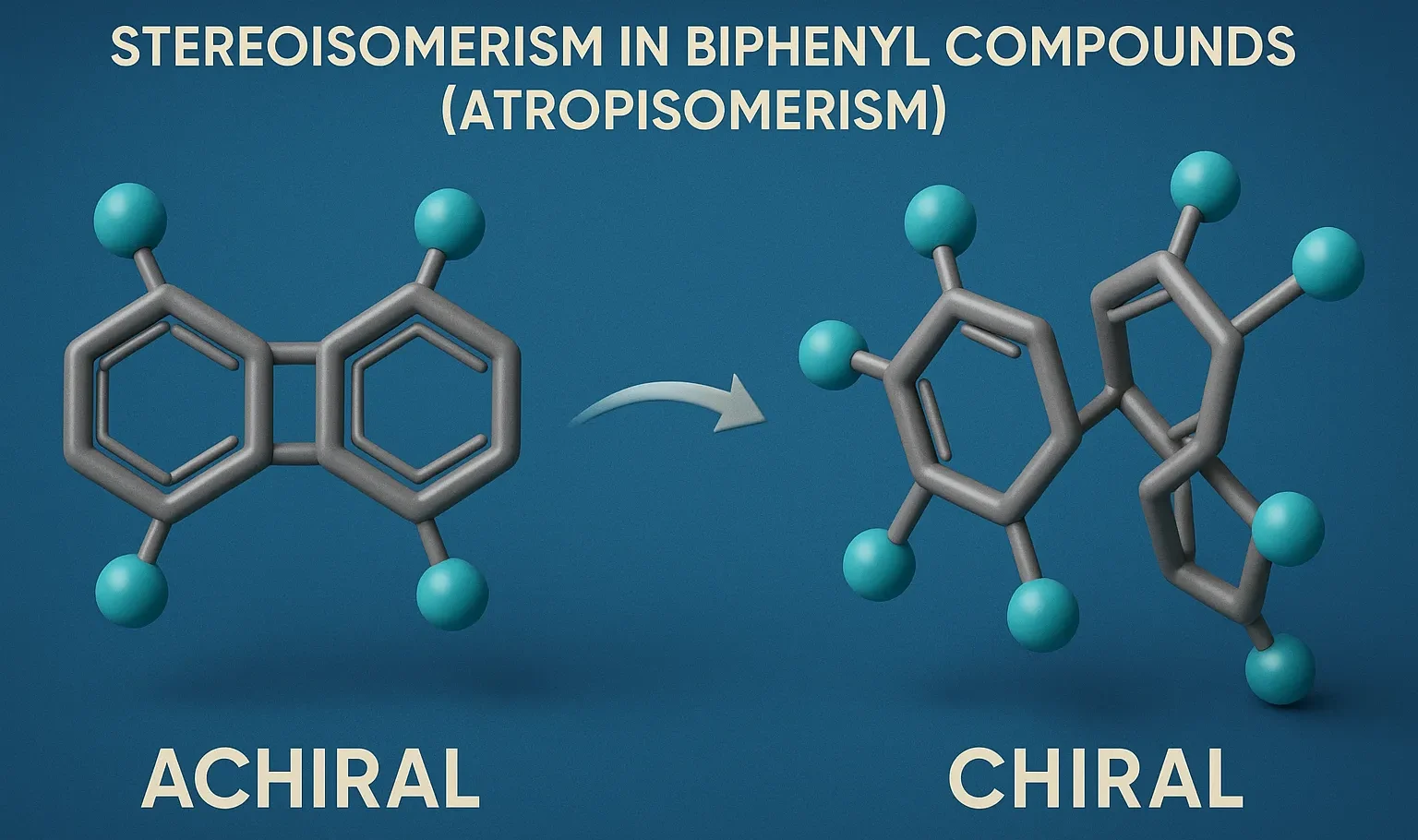
Stereoisomerism in Biphenyl Compounds (Atropisomerism) occurs when restricted rotation around the biphenyl bond creates stable, isolable isomers with distinct properties.
What is Biphenyl?
- Biphenyl is a molecule made of two benzene rings joined by a single bond:
- The two rings can rotate around the central C–C bond.
- However, rotation can be restricted if large groups are present at the ortho positions (positions 2 and 2′, next to the connecting bond).
Advertisements
Atropisomerism
- Atropisomerism is a form of axial stereoisomerism caused by restricted rotation around a single bond due to steric hindrance.
- Results in stable enantiomers (non-superimposable mirror images).
- The term comes from Greek: a-tropos = “without turning”.
- Commonly observed in ortho-substituted biphenyls.
Conditions for Atropisomerism in Biphenyls
-
Bulky Ortho Substituents
- Sterically hinder rotation.
- Examples: –NO₂, –COOH, –Br, –t-butyl, –OH, –NH₂
-
High Rotational Barrier
- Barrier ≥ 100 kJ/mol allows isolation of enantiomers.
- Lower barriers result in rapid interconversion.
-
Lack of Internal Symmetry
- Must lack a plane of symmetry to be chiral.
Advertisements
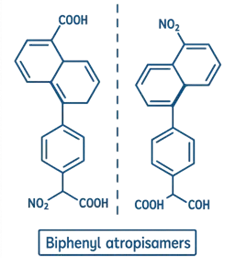
Example
Click Here to Watch the Best Pharma Videos
Advertisements