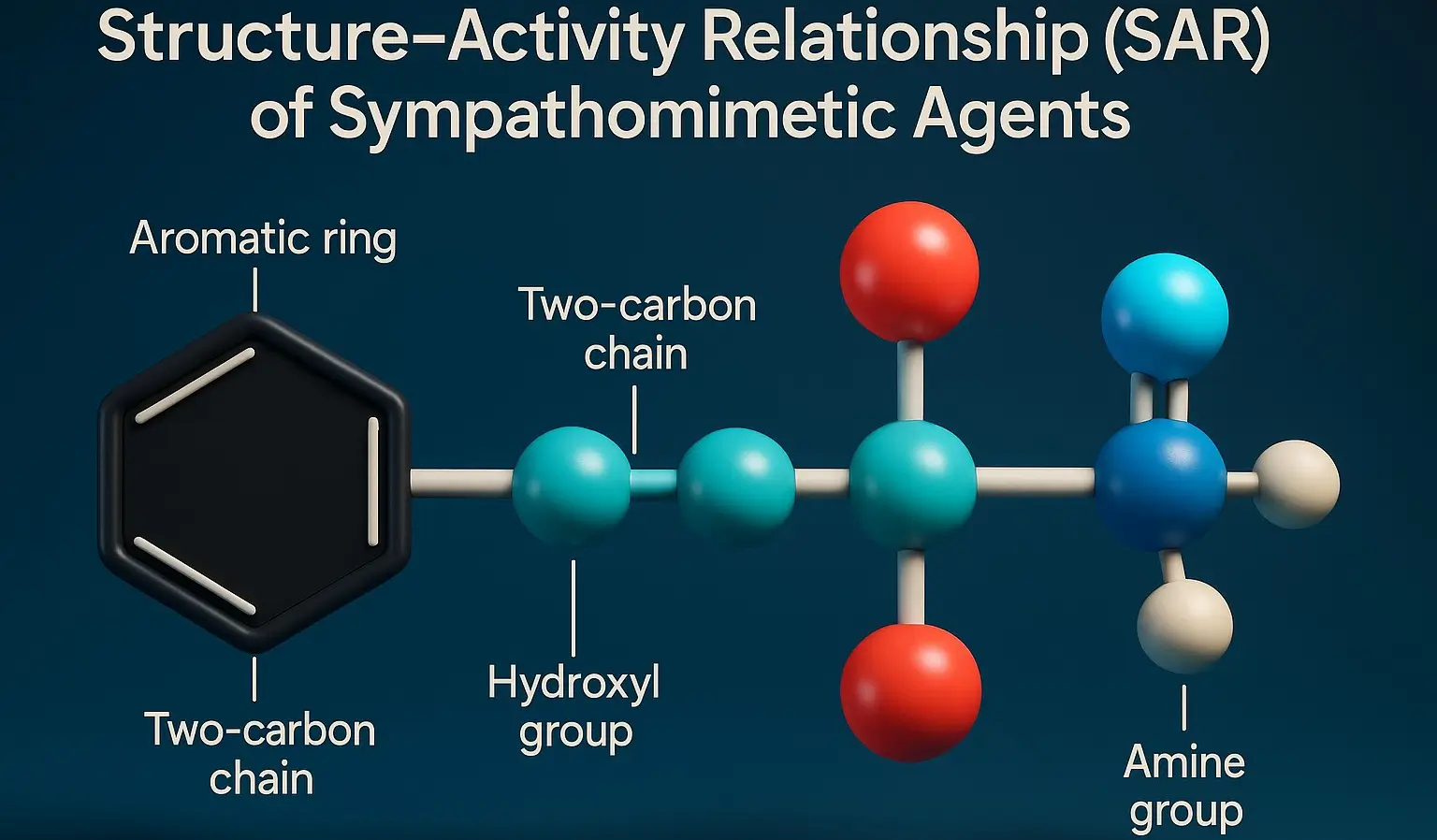
- Structure-Activity Relationship mimic catecholamines (epinephrine, norepinephrine, dopamine) by stimulating adrenergic receptors (α and β).
- Their activity depends on structural modifications affecting receptor selectivity, metabolism, and CNS penetration.
Key Structure-Activity Relationship Features
-
Catechol Ring Substitutions
- 3,4-Dihydroxy (Catechol): High α/β activity, rapid metabolism (e.g., Epinephrine, Norepinephrine).
- Single Hydroxyl (-OH) Group: Reduces metabolism, increases α1 selectivity (e.g., Phenylephrine).
- No -OH Groups: Poor COMT metabolism, better CNS penetration (e.g., Amphetamine, Ephedrine).
-
Amine Group Substitutions
- Small groups (-H, -CH3): Preferential α-receptor activity (e.g., Norepinephrine).
- Bulky groups (-CH(CH3)2, -C(CH3)3): Enhanced β-selectivity (e.g., Isoproterenol, Albuterol).
-
Alpha-Carbon (-CH3) Substitution
- Inhibits MAO metabolism, prolongs duration, increases CNS effects (e.g., Ephedrine, Amphetamine).
-
Beta-Hydroxyl Group (-OH at β-carbon)
- Present: Strong receptor binding, reduced CNS penetration (e.g., Epinephrine).
- Absent: Increased CNS activity, indirect action (e.g., Amphetamine).
Classification Based on SAR
- Direct-Acting: Epinephrine (α/β), Phenylephrine (α1), Albuterol (β2).
- Indirect-Acting: Amphetamine, Tyramine, Cocaine.
- Mixed-Acting: Ephedrine, Pseudoephedrine.
Click Here to Watch the Best Pharma Videos