Synthesis of Acridine covers Bernthsen condensation, Ullmann coupling, and intramolecular cyclization for dyes and drug discovery.
-
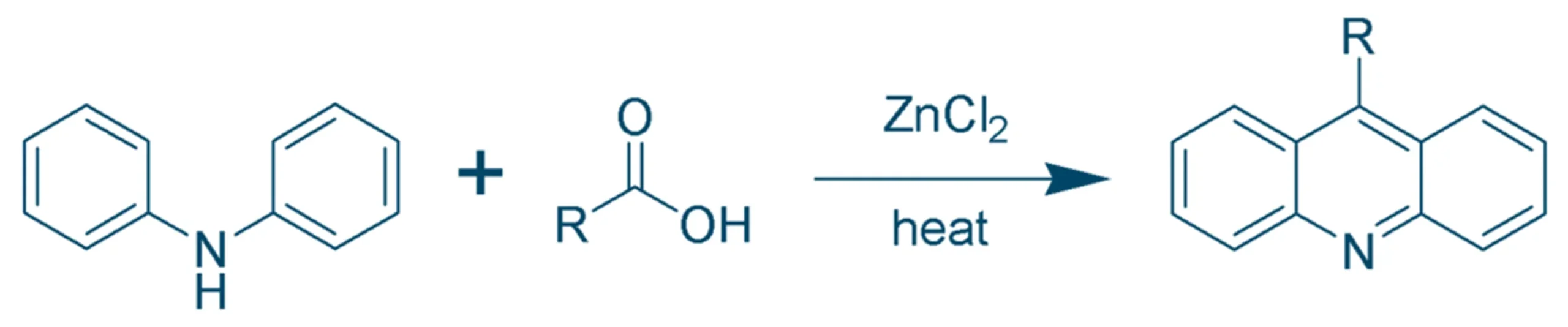
Bernthsen Synthesis (Classical Method)
- Reactants: Diphenylamine + Carboxylic acid (or acid anhydride)
- Catalyst: Zinc chloride (ZnCl₂)
- Conditions: High temperature (~250–270 °C)
- Reaction:
-
- Diphenylamine + Formic acid → Acridine + Water
-

-
- Mechanism: Involves Friedel–Crafts acylation, cyclization, and dehydration.
-
From Anthranilic Acid Derivatives
- Anthranilic acid derivatives condense with aldehydes to give acridone intermediates.
- Reduction of acridone → Acridine
-
Industrial Methods
- Condensation of o-nitrobenzaldehyde with N,N-diarylamines, followed by reduction and cyclization.