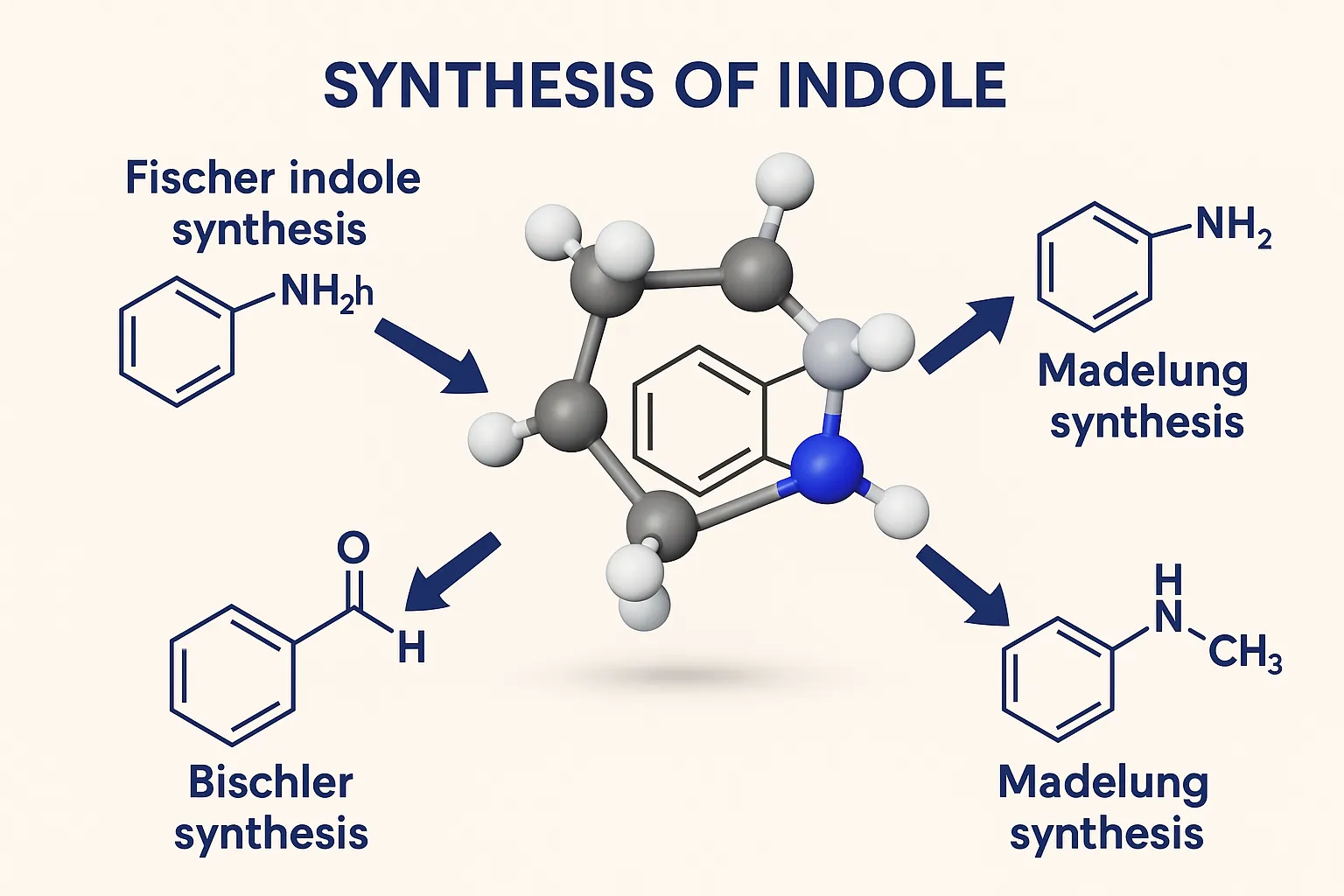
Synthesis of Indole covers Fischer indole, Madelung, Bartoli, and Nenitzescu methods with key steps for medicinal chemistry.
-
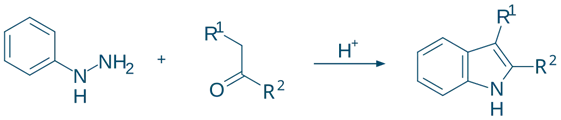
Fischer (Most common method)
- Reactants: Phenylhydrazine + Aldehyde or Ketone
- Conditions: Acidic, heat
- Example:
- Phenylhydrazine + Acetone → Indole (after cyclization and rearrangement)

- Mechanism:
- Hydrazone formation
- [3,3]-Sigmatropic rearrangement (Fischer)
- Cyclization and aromatization
-
Bischler–Möhlau Indole Synthesis
- Reactants: Aniline + α-haloketone
- Conditions: Heat
- Reaction:
-
- Aniline + α-haloketone → Indole derivative
- Used for substituted indoles
-
-
Madelung Synthesis
- Reactants: o-Toluidine + Acid chloride (intramolecular cyclization)
- Reagents: Base (e.g., NaOMe), high temperature
-
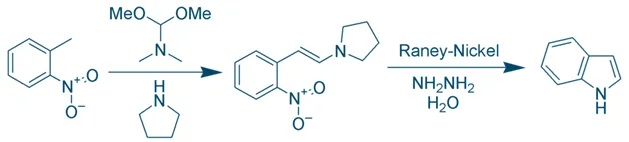
Leimgruber–Batcho Synthesis
- Used in pharmaceutical industry for high-yield synthesis of substituted indoles