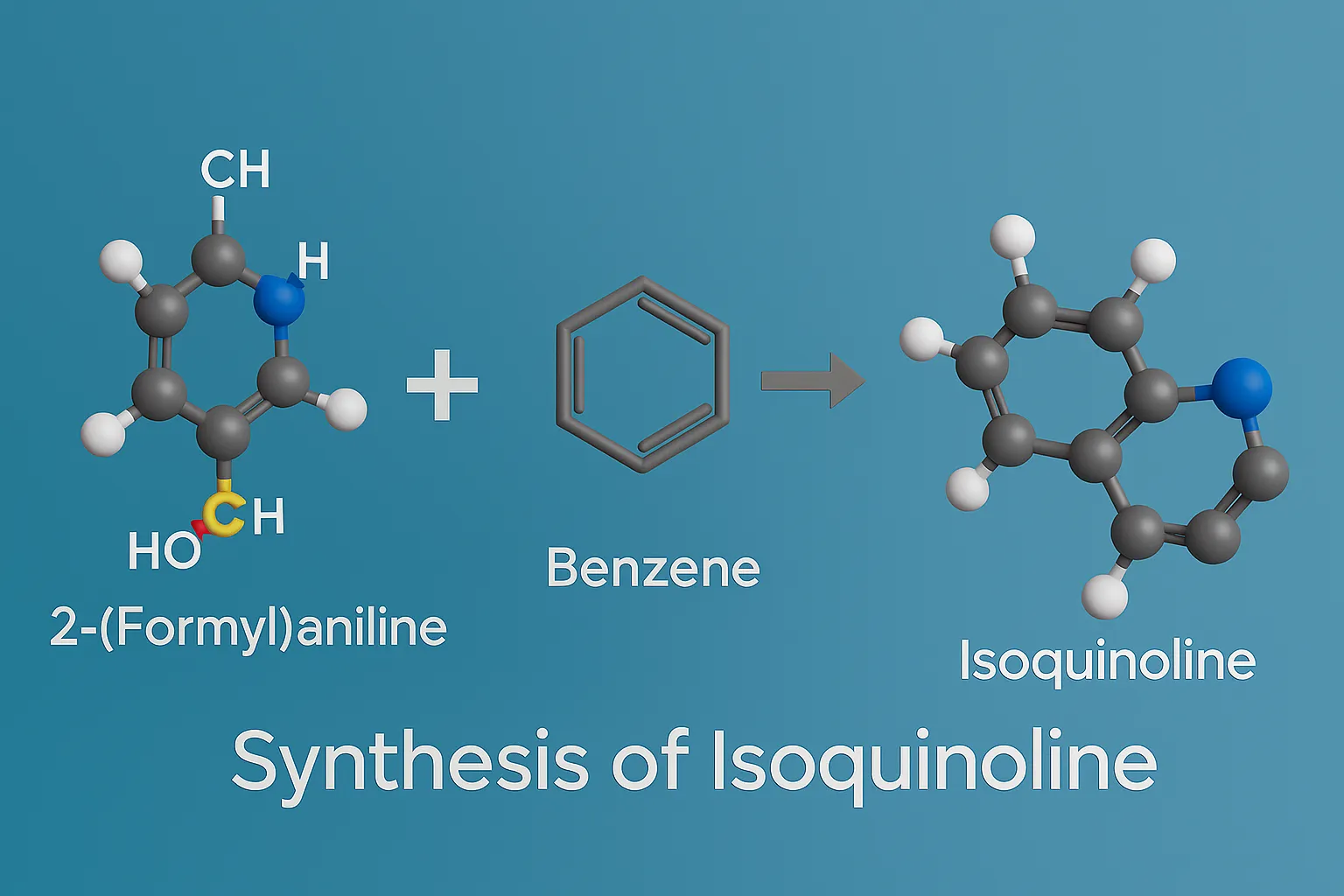
Synthesis of Isoquinoline covers Bischler–Napieralski, Pictet–Spengler, and Pomeranz–Fritsch routes with key steps for drug design.
-
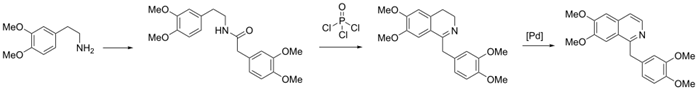
Bischler–Napieralski Synthesis
- Reactants: β-Phenylethylamine + acyl chloride → Cyclization
- Reagents: POCl₃ or P₂O₅ (dehydrating agents)
- Steps:
- Acylation of phenylethylamine
- Cyclization under acidic conditions
- Dehydrogenation → Isoquinoline

-
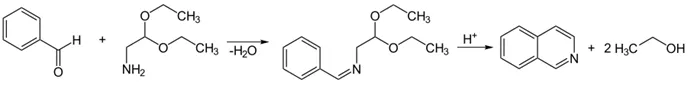
Pomeranz–Fritsch Reaction
- Reactants: Benzaldehyde + aminoacetaldehyde diethyl acetal
- Conditions: Acidic cyclization
- Reaction:
-
- Benzaldehyde + H₂NCH₂CH(OEt)₂ → Isoquinoline derivative

- Benzaldehyde + H₂NCH₂CH(OEt)₂ → Isoquinoline derivative
- Useful for simple unsubstituted or mono-substituted isoquinolines
-