- Theories of Emulsification aid in formulating stable pharmaceutical and food emulsions.
- Theories of Emulsification explain how emulsifying agents stabilize oil-water mixtures.
- These are theories that explain the formation and stabilization of emulsions:
-
Monomolecular Theory
- Emulsifying agents like surfactants form a monomolecular film at the oil-water interface.
- These molecules have hydrophilic (water-loving) and lipophilic (oil-loving) ends, aligning themselves at the interface.
- The film reduces interfacial tension and stabilizes droplets.
-
Multimolecular Theory
- Applies to emulsifiers like gums or proteins.
- These form multilayer films around dispersed droplets.
- The thicker the film, the better the mechanical barrier against coalescence, thus improving stability.
-
Solid Particle Theory
- Involves the use of finely divided solid particles (e.g., bentonite, veegum).
- These particles adsorb at the interface and form a coating around droplets, preventing them from merging.
- Stability is due to the physical barrier created by the solid particles.
Advertisements
Other Common Theories (as previously noted):
-
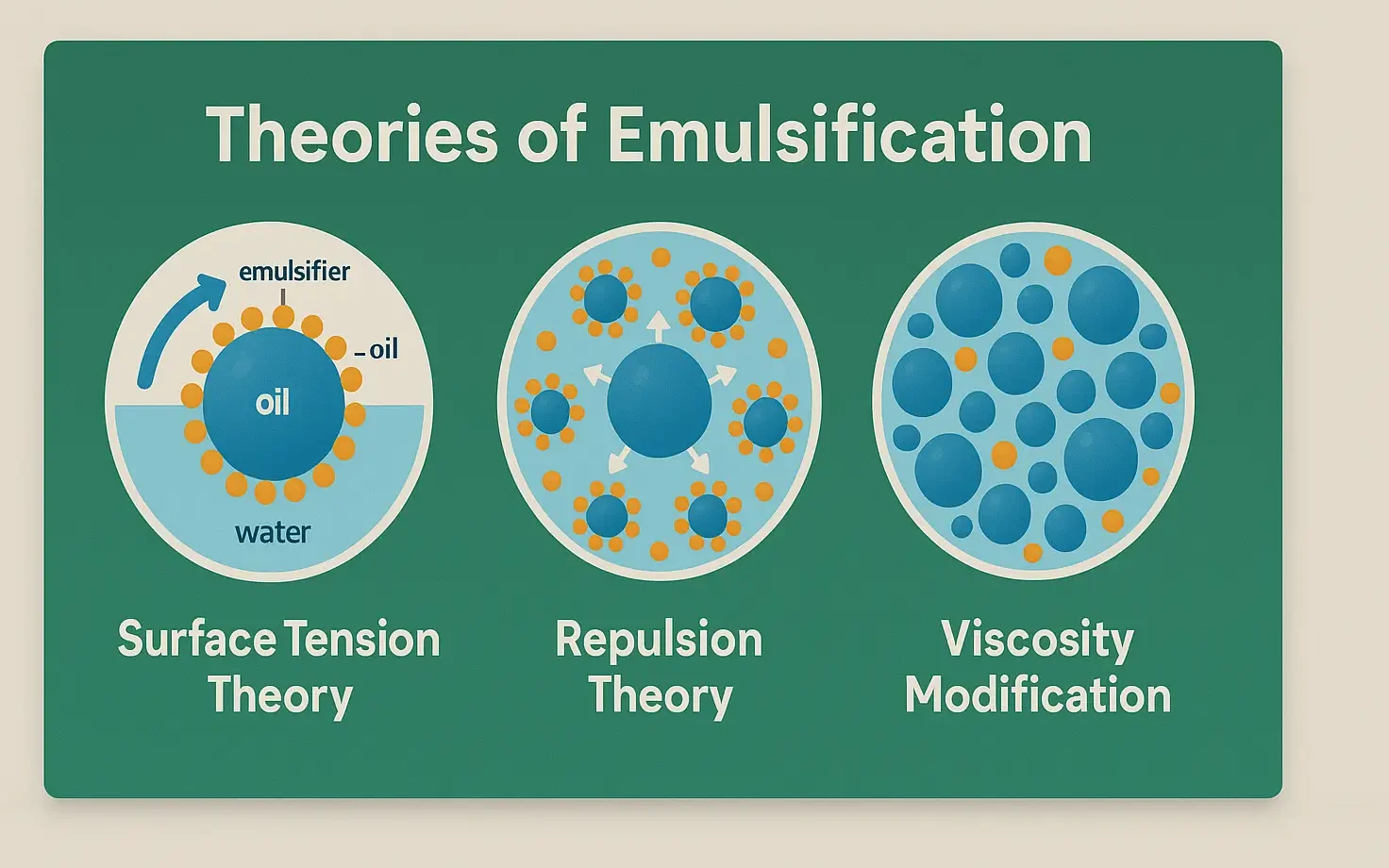
Surface Tension Theory
- Emulsification occurs by reducing interfacial tension using surfactants.
-
Oriented Wedge Theory
- The shape and orientation of emulsifier molecules determine the type of emulsion formed (O/W or W/O).
-
Interfacial Film Theory
- Emphasizes the strength and stability of films formed by emulsifiers around droplets.
-
Hydration Theory
- Focuses on the water-absorbing and swelling behavior of emulsifiers that create viscosity and stability.
Click Here to Watch the Best Pharma Videos
Advertisements