- Thermodynamic Treatment of Stability constants quantify the strength of the complex formed between a central atom and ligands.
- Understanding their thermodynamics is crucial for predicting complex behavior in various conditions.
Stability Constants (Formation Constants)
- Definition: Equilibrium constants representing the formation of a complex from its components.
- Notation: K_f = [Complex]ⁿ / ([Metal]ᵐ × [Ligand]ᵖ)
-
Types:
- Stepwise Stability Constants: Ki for the formation of each additional ligand.
- Overall Stability Constant: β_n for the formation of the complex with n ligands.
Factors Affecting Stability Constants
- Charge of the Metal Ion: Higher charges generally increase stability.
- Chelate Effect: Polydentate ligands form more stable complexes than equivalent monodentate ligands.
- Ligand Basicity: Stronger donor atoms enhance stability.
- Steric Factors: Bulky ligands may hinder complex formation.
- Solvent Effects: Polar solvents can stabilize charged complexes.
Thermodynamics of Complex Formation
-
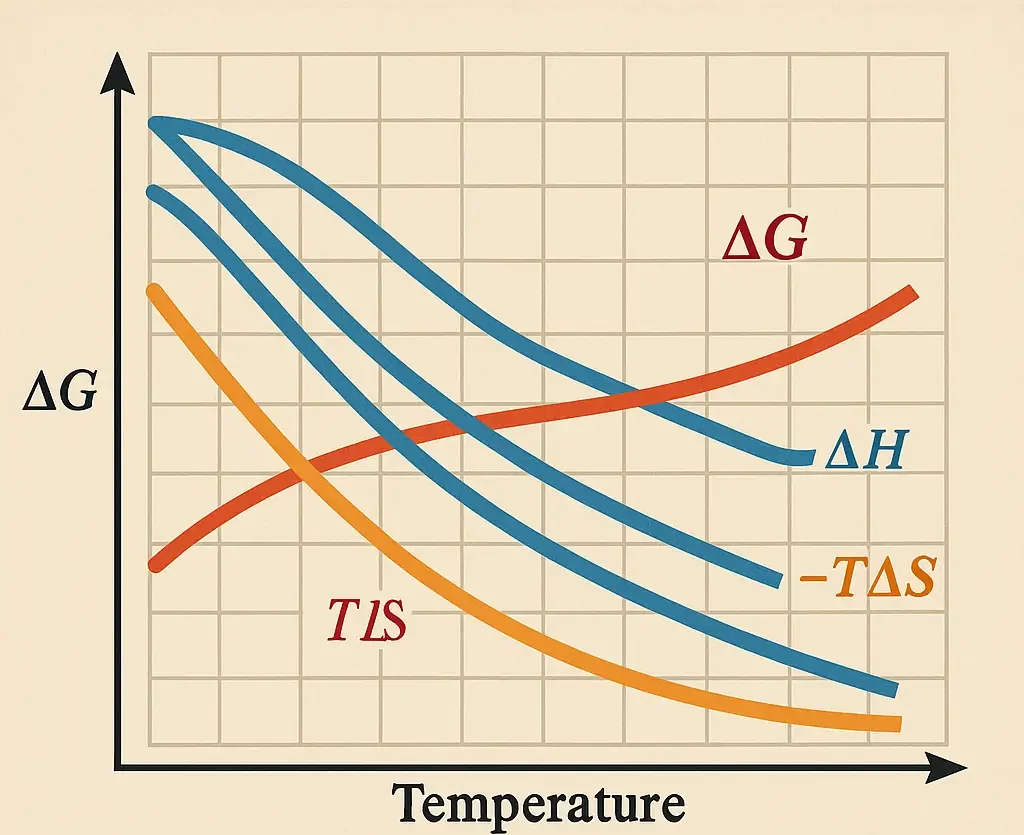
Gibbs Free Energy (ΔG):
-
$\Delta G = -RT \ln K_f$
- Negative ΔG indicates spontaneous complex formation.
-
Enthalpy (ΔH):
- Heat change during complex formation.
- Exothermic (negative ΔH) often correlates with stronger binding.
-
Entropy (ΔS):
- Disorder change upon complexation.
- Complex formation can be driven by entropy gains (e.g., release of water molecules) or losses (e.g., ordering of ligands).
Applications of Stability Constants
- Predicting Complex Behavior: Determines which complexes will form under given conditions.
- Designing Chelating Agents: Guides the selection of ligands for specific metal ions.
- Environmental Chemistry: Assesses the mobility and bioavailability of metal contaminants.
- Pharmaceuticals: Optimizes drug-protein binding and drug delivery systems
Click Here to Watch the Best Pharma Videos