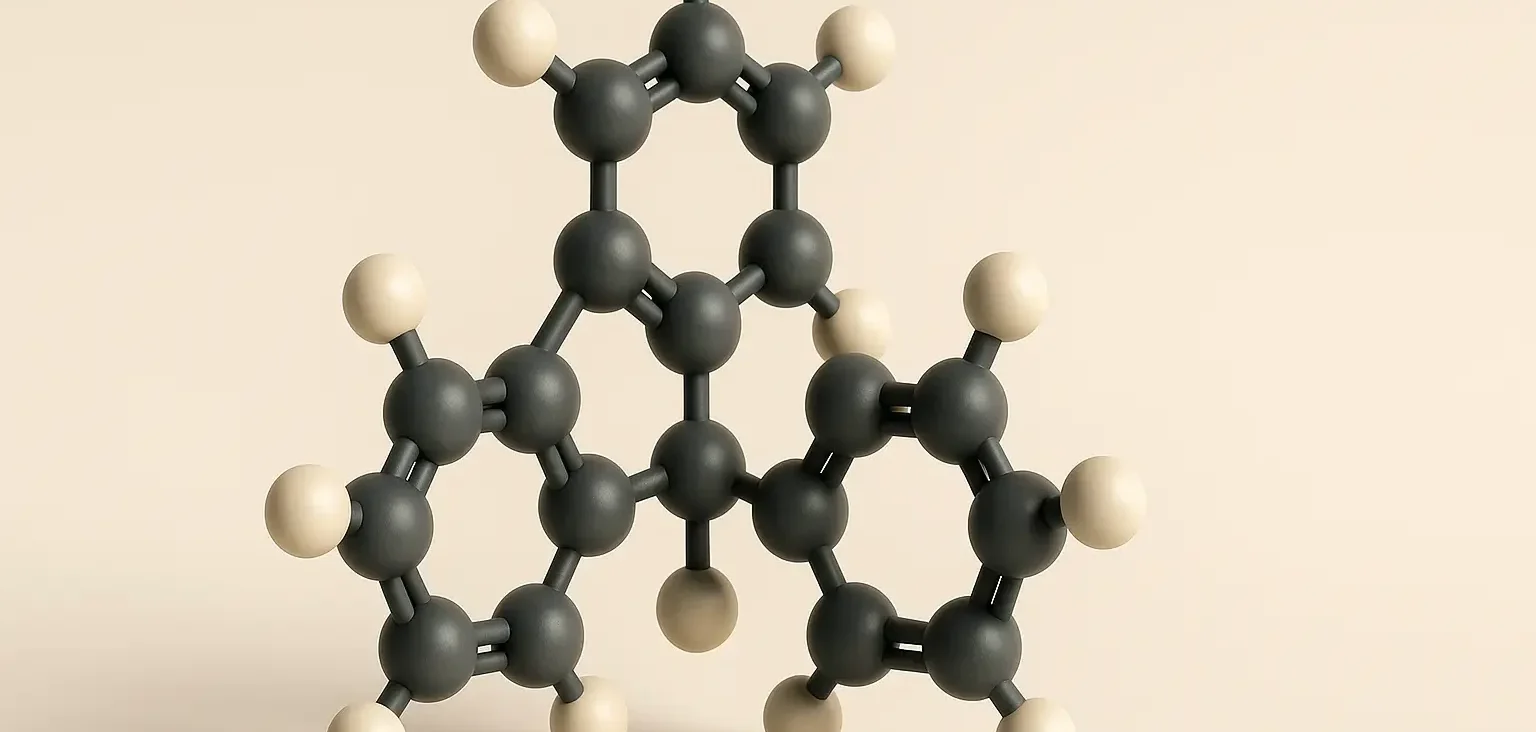
Structure of Triphenylmethane:
- Triphenylmethanes have a central carbon atom bonded to three phenyl groups. Its molecular formula is C19H16.
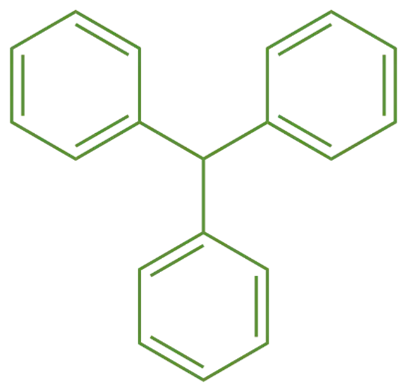
Synthesis of Triphenylmethane:
-
Friedel-Crafts Alkylation:
- Reaction: Benzene reacts with chloroform in the presence of aluminum chloride to form triphenylmethanes.
-
$\mathrm{3C_6H_6 + CHCl_3 \xrightarrow{AlCl_3} C(C_6H_5)_3 + 3HCl}$
-
- Reaction: Benzene reacts with chloroform in the presence of aluminum chloride to form triphenylmethanes.
-
Grignard Reaction:
- Reaction: Benzophenone reacts with phenylmagnesium bromide to form triphenylmethanes.
-
$\mathrm{C_6H_5COC_6H_5 + C_6H_5MgBr \rightarrow C(C_6H_5)_3 + MgBrOH}$
-
- Reaction: Benzophenone reacts with phenylmagnesium bromide to form triphenylmethanes.
Reactions of Triphenylmethane:
-
Oxidation:
- Triphenylmethanes can be oxidized to triphenylmethyl carbocation, which is a colored intermediate in the formation of triphenylmethanes dyes.
-
$\mathrm{C(C_6H_5)_3 \rightarrow [O]C^+ (C_6H_5)_3 + e^-}$
-
- Triphenylmethanes can be oxidized to triphenylmethyl carbocation, which is a colored intermediate in the formation of triphenylmethanes dyes.
-
Electrophilic Substitution:
- Nitration: Triphenylmethane can be nitrated to produce nitrotriphenylmethane.
-
$\mathrm{C(C_6H_5)_3 + HNO_3 \xrightarrow{H_2SO_4} NO_2C_6H_4C(C_6H_5)_2 + H_2O}$
-
- Nitration: Triphenylmethane can be nitrated to produce nitrotriphenylmethane.
Advertisements
Medicinal Uses:
- Dyes: Many triphenylmethanes derivatives are used as dyes, some of which have been used in medicine. For example, gentian violet (triphenylmethanes dye) is used as an antiseptic.
- Other Derivatives: Some derivatives of triphenylmethanes have been explored for their potential as antitumor and antimicrobial agents.