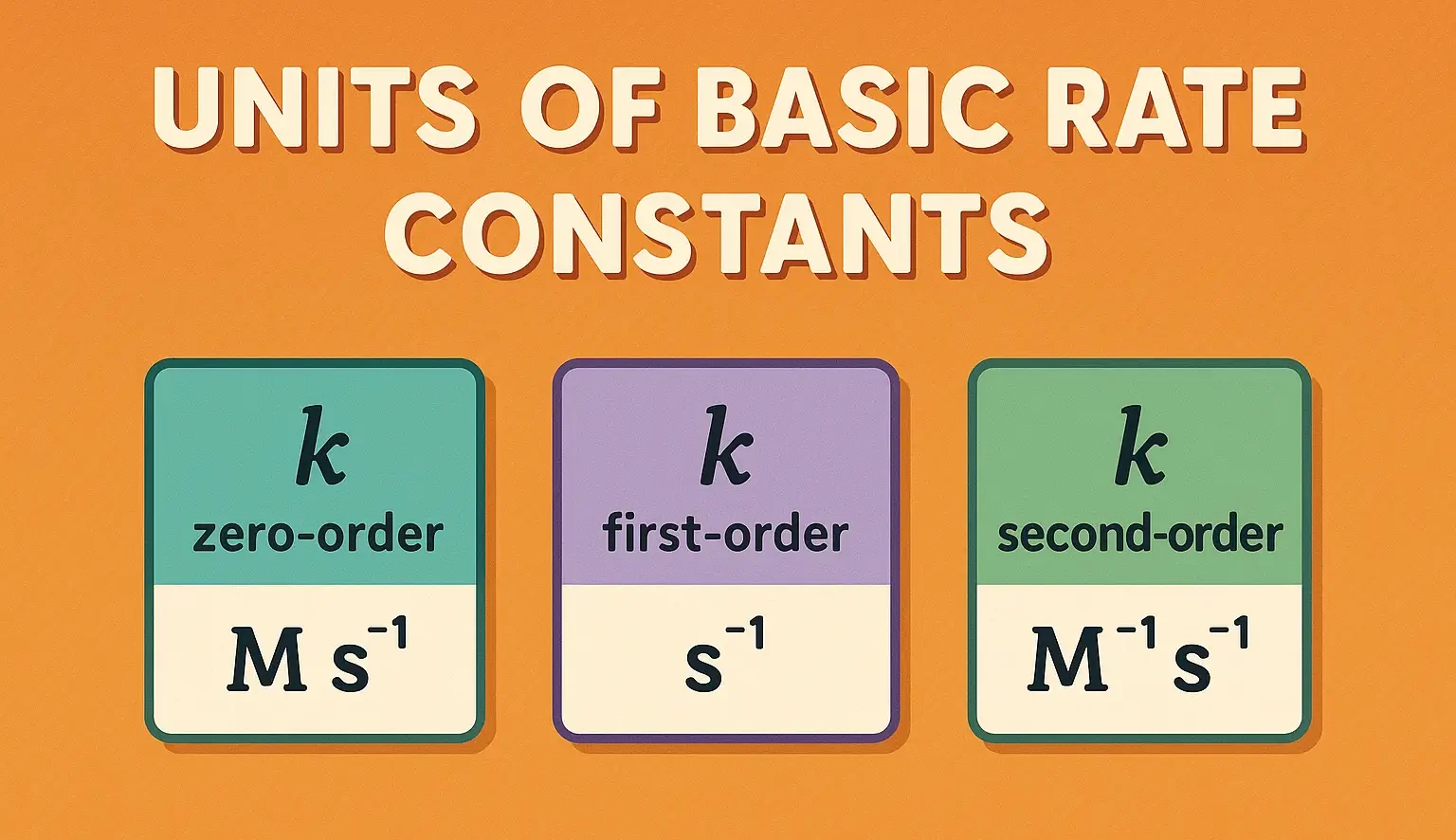
The units of k depend on the reaction order. To ensure the rate has units of concentration/time (e.g., mol/L·s), units of k vary:
| Reaction Order | Rate Expression | Units of k |
| Zero | Rate = k | mol·L⁻¹·s⁻¹ or concentration/time |
| First | Rate = | s⁻¹ or time⁻¹ |
| Second | Rate = | L·mol⁻¹·s⁻¹ or concentration⁻¹·time⁻¹v |