- Zero-Order Reactions explain drug elimination like alcohol and phenytoin at saturating doses.
- Definition: Rate of degradation is independent of concentration.
- Rate law:
\(\text{Rate} = -\frac{d[A]}{dt} = k\)
Separate variables:
\( d[A] = -k \, dt \)
Integrate both sides:
$\int_{[A]_0}^{[A]} d[A] = -k \int_{0}^{t} dt$
- Graph: Plot of [A] vs. time = straight line with slope = -k
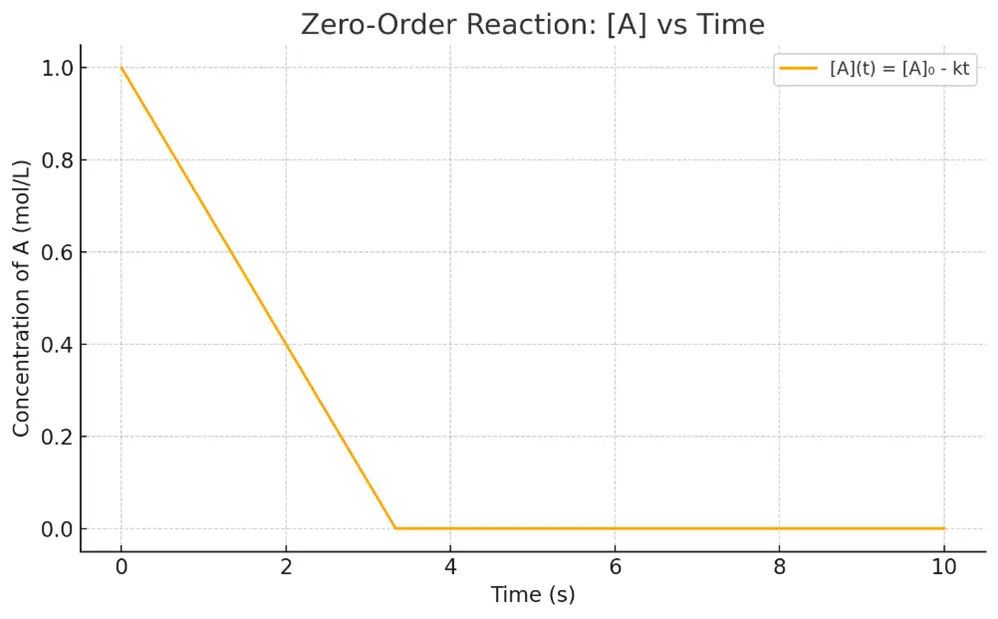
- Example: Decomposition of drug in a suspension (saturated solution), where concentration remains constant.
Features:
- Constant degradation per unit time
- Half-life depends on initial concentration:
$t_{1/2} = \frac{[A]_0}{2k}$
- Shelf life (t90t_{90}t90):
$t_{90} = \frac{0.1 [A]_0}{k}$