E1 and E2 reaction Definition
- E1 and E2 are elimination reactions in organic chemistry, where atoms or groups are removed, leading to the formation of a double bond.
- Their primary difference lies in their reaction mechanisms.
E1 Reaction (Unimolecular Elimination)
- Two-step mechanism involving a carbocation intermediate.
- The rate depends only on the substrate (R−LGR-LGR−LG).
This is a sample ad placement!
Mechanism:
-
Carbocation Formation (Slow Step)
- The leaving group (LG) departs, forming a carbocation.
- Equation: R−LG → R+ + LG−
-
Proton Abstraction and Double Bond Formation (Fast Step)
- A base removes a proton (β-hydrogen) from a carbon adjacent to the carbocation, forming an alkene.
- Equation: R+ + B− → R=CR2 + BH+
Example:
-
Dehydration of 2-Methyl-2-Butanol:
- Step 1: Protonation of the alcohol (-OH) turns it into a good leaving group (water).
- Step 2: The loss of water forms a tertiary carbocation.
- Step 3: A base abstracts a proton, forming 2-methyl-2-butene.
This is a sample ad placement!
Factors Influencing E1 Reactions:
- Carbocation Stability: Tertiary, allylic, or benzylic carbocations are more stable and favor E1 reactions.
- Leaving Group: A good leaving group facilitates carbocation formation.
- Solvent: Polar solvents stabilize the carbocation and leaving group.
- Temperature: Higher temperatures favor E1 reactions by providing energy for carbocation formation.
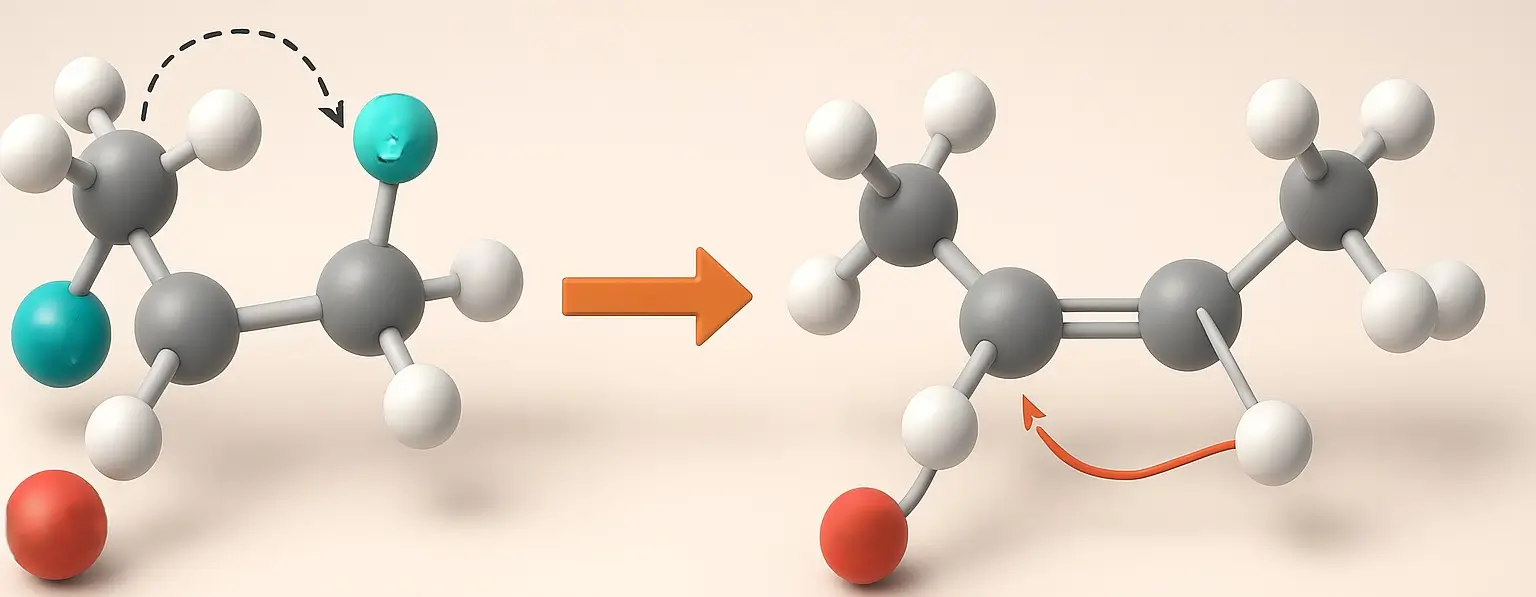
E2 Reaction (Bimolecular Elimination)
- The E2 reaction is a one-step process where the leaving group is eliminated, and a proton is abstracted simultaneously.
This is a sample ad placement!
Mechanism:
- A strong base abstracts a β-hydrogen, and the electrons from the C-H bond form a double bond while ejecting the leaving group.
- This process is bimolecular, with the rate depending on both substrate and base concentrations.
- The reaction often follows anti-periplanar geometry, where the hydrogen and leaving group are on opposite sides.
Example:
- 1-Bromopropane and Hydroxide Ion Reaction:
- Equation: CH₃CH₂CH₂Br + OH⁻ → CH₃CH=CH₂ + Br⁻ + H₂O
- Here, hydroxide abstracts a β-hydrogen, forming propene, bromide, and water.
Thank you for reading from Firsthope's notes, don't forget to check YouTube videos!
This is a sample ad placement!