- Conjugated Dienes Preparing Methods are organic compounds containing two double bonds separated by a single bond, allowing electron delocalization.
- Conjugated Dienes Preparing Methods are important intermediates in various organic reactions, such as Diels-Alder reactions.
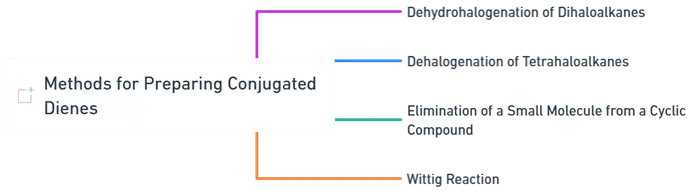
- Below are some key methods for preparing conjugated dienes:
This is a sample ad placement!
Dehydrohalogenation of Dihaloalkanes
- Mechanism: This is an elimination reaction where both a hydrogen atom and a halogen atom are removed from a dihaloalkane, forming a conjugated diene.
- Conditions: Requires a strong base, such as an alkoxide ion or hydroxide ion.
- General Reaction: R-CH=CH-CH2-X + B- → R-CH=CH-CH=CH2 + HB + X-
- Example:
- Synthesis of 1,3-Butadiene from 1,4-Dichlorobutane:
- CH2Cl-CH2-CH=CH2 + 2 KOH → CH2=CH-CH=CH2 + 2 KCl + 2 H2O
Dehalogenation of Tetrahaloalkanes
- Mechanism: Involves the removal of two halogen atoms from a tetrahaloalkane. This process leads to the formation of a conjugated diene.
- Conditions: Often requires a metal such as magnesium or zinc, used in the presence of a suitable solvent.
- General Reaction: R-CHX-CHX-CHX-CHX-R + 2 M → R-CH=CH-CH=CH-R + 2 MX2
- Example:
- Synthesis of 1,3-Butadiene from 1,4-Dibromo-2,3-Dichlorobutane:
- CH2Br-CHCl-CHCl-CH2Br + 2 Mg → CH2=CH-CH=CH2 + 2 MgBr2 + 2 MgCl2
This is a sample ad placement!
Elimination of a Small Molecule from a Cyclic Compound
- Mechanism: Conjugated dienes can also be formed by the elimination of small molecules (e.g., water, hydrogen chloride) from cyclic compounds.
- Conditions: Typically involves the use of heat or a suitable catalyst.
- General Reaction: Cyclic compound → Conjugated diene + Small molecule
- Example:
- Preparation of 1,3-Cyclohexadiene from Cyclohexene Oxide:
- Cyclohexene oxide (heating) → 1,3-cyclohexadiene + H2O
Wittig Reaction
- Mechanism: A method for preparing alkenes (including conjugated dienes) from aldehydes or ketones using a phosphonium ylide.
- Conditions: Suitable for aldehydes or ketones containing an α,β-unsaturated carbonyl group.
- General Reaction: R1-CH=O + R2P=CHR3 → R1-CH=CHR3 + R2P=O
- Example:
- Synthesis of 1,3-Butadiene from Acrolein and Methylenetriphenylphosphorane:
- CH2=CH-CH=O + Ph3P=CH2 → CH2=CH-CH=CH2 + Ph3P=O
This is a sample ad placement!
Thank you for reading from Firsthope's notes, don't forget to check YouTube videos!