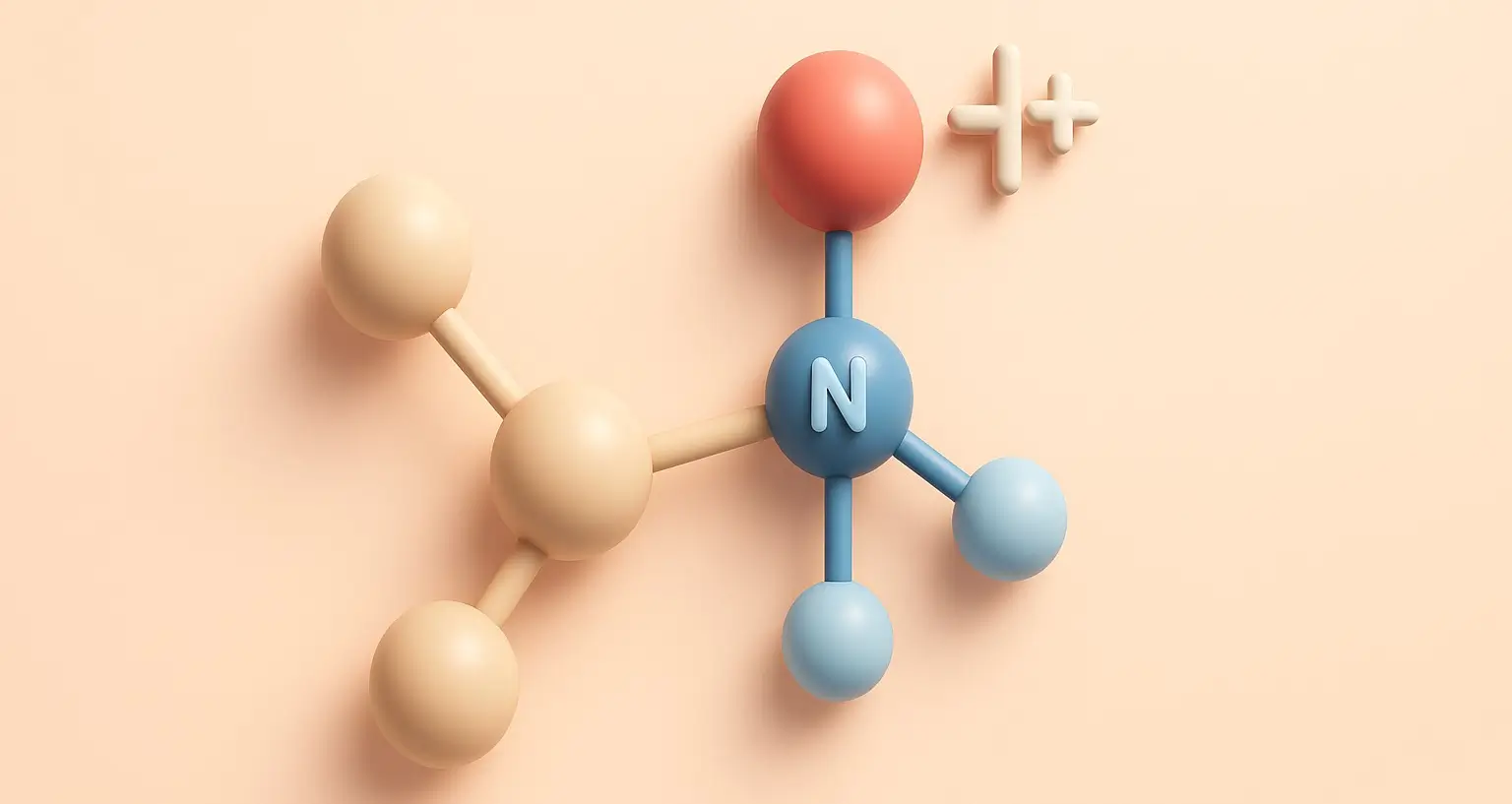
Basicity of Aliphatic Amines Definition
- Basicity is a measure of a compound’s ability to accept protons (H⁺).
- In aliphatic amines, arises from the lone pair of electrons on the nitrogen atom, which can accept a proton, forming a positively charged ammonium ion.
Quantifying
- The basicity of amines is often expressed through their pKb values, which are derived from the base dissociation constant (Kb).
- pKb values: A lower pKb indicates a stronger base, while a higher pKb signifies a weaker base.
Effect of Substituents
- Its aliphatic amines is influenced by the nature and positioning of substituents on or near the nitrogen atom. Key factors include:
-
Alkyl Groups:
- Electron-Donating Effect: Alkyl groups attached to nitrogen exert an inductive effect, pushing electron density towards the nitrogen, which enhances the nitrogen’s ability to donate its lone pair and increases the amine’s basicity.
- Order of Basicity: The basicity generally increases with the number of alkyl groups in the order: primary (1°) < secondary (2°) < tertiary (3°) amines.
-
Electron-Withdrawing Groups (EWGs):
- EWGs, such as halogens or nitro groups, pull electron density away from the nitrogen atom, reducing the electron density available for protonation and thus decreasing the amine’s basicity.
-
Steric Hindrance:
- Bulky substituents near the nitrogen can physically obstruct the approach of protons, making it more difficult for the amine to accept a proton. This steric hindrance reduces the basicity’s, especially in tertiary amines with large alkyl groups.
-
Hybridization of the Nitrogen Atom:
- sp³ vs. sp² Hybridization: Amines with sp³-hybridized nitrogen are generally more basic than those with sp²-hybridized nitrogen. The higher s-character in sp² hybridization pulls the lone pair closer to the nitrogen, making it less available for protonation, thereby reducing.
- These factors collectively determine the aliphatic amines, influencing their behavior in chemical reactions and interactions.
Thank you for reading from Firsthope's notes, don't forget to check YouTube videos!