Chloroform (Trichloromethane) Definition
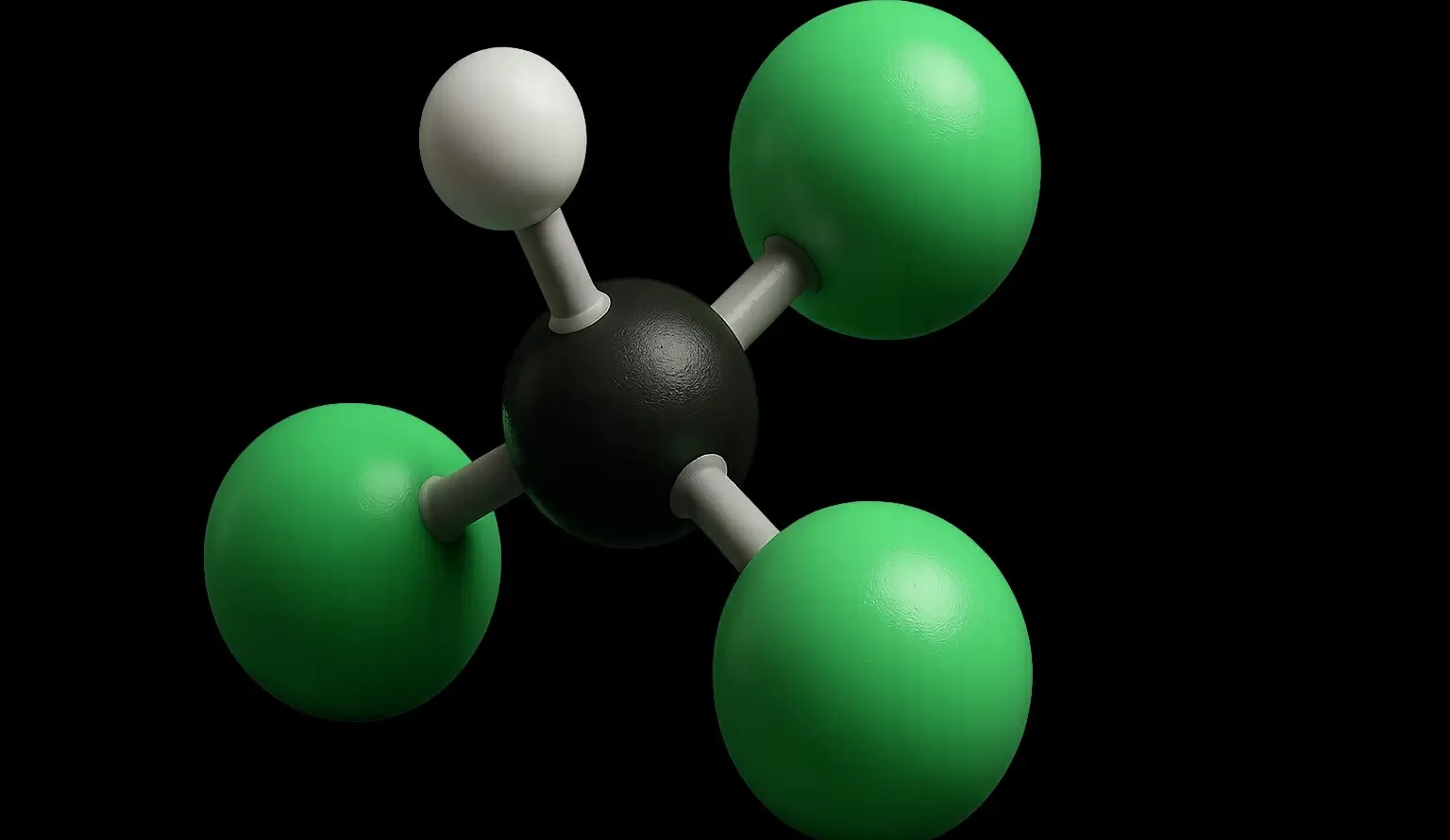
- Chloroform (Trichloromethane), also known by its IUPAC name trichloromethane (CHCl₃), is a colorless, volatile, and sweet-smelling liquid that is non-flammable and heavier than water.
- It belongs to the class of compounds called halogenated hydrocarbons and is primarily used as a solvent, reagent, and intermediate in the production of various chemicals, especially refrigerants and fluorocarbons.
Advertisements
Structure of Chloroform:
- Chemical Formula: CHCl₃
- Molecular Structure: Comprises a single carbon atom bonded to one hydrogen atom and three chlorine atoms.
- Bonding: The carbon atom forms four single bonds—one with hydrogen and three with chlorine.
- Geometry: Tetrahedral, with chlorine atoms having a greater spatial distribution due to their larger size compared to hydrogen.
Uses:
- Anesthetic: Historically used as an inhalation anesthetic in medicine, though it’s less common now due to safety concerns.
- Solvent: Utilized in the laboratory as a solvent for fats, oils, rubber, alkaloids, and resins.
- Intermediate: Used in the production of refrigerants and fluoropolymers.
- Pesticide: Sometimes used as a fumigant for grains and crops.
Advertisements