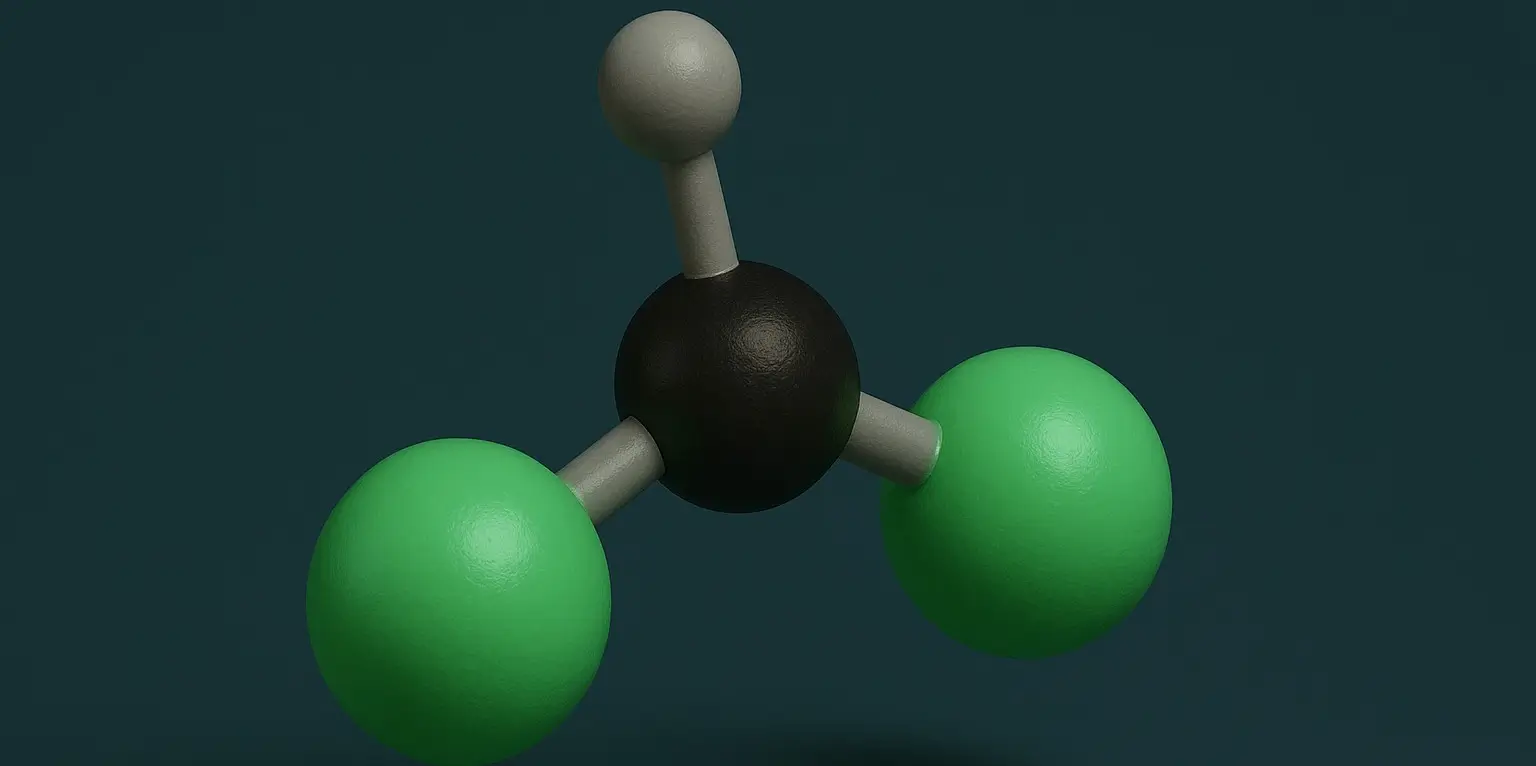
Dichloromethane (Methylene Chloride) Definition
- Dichloromethane (Methylene Chloride), also known as methylene chloride, is a volatile, colorless liquid with a moderately sweet aroma.
- It is a halogenated hydrocarbon with the chemical formula:
Structure:
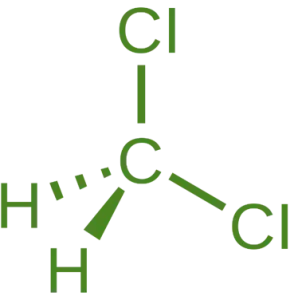
- Chemical Formula: CH₂Cl₂
- Molecular Structure: A single carbon atom bonded to two hydrogen atoms and two chlorine atoms.
- Bonding: The carbon atom forms four single bonds, with two bonds to hydrogen and two to chlorine.
- Geometry: Tetrahedral around the carbon atom.
Uses:
- Solvent: Commonly used as a solvent in the pharmaceutical and chemical industries due to its ability to dissolve a wide range of organic compounds.
- Paint Stripping: Used in paint removers and degreasers.
- Extraction: Employed in the extraction of certain chemicals due to its volatility and solvent properties.
Advertisements