- Limit tests is an analytical method used to determine whether the concentration of a specific impurity or substance in a pharmaceutical product is below a predefined acceptable limit.
- It ensures the quality, safety, and efficacy of the drug by controlling potentially harmful impurities.
Types of Limit Tests:
-
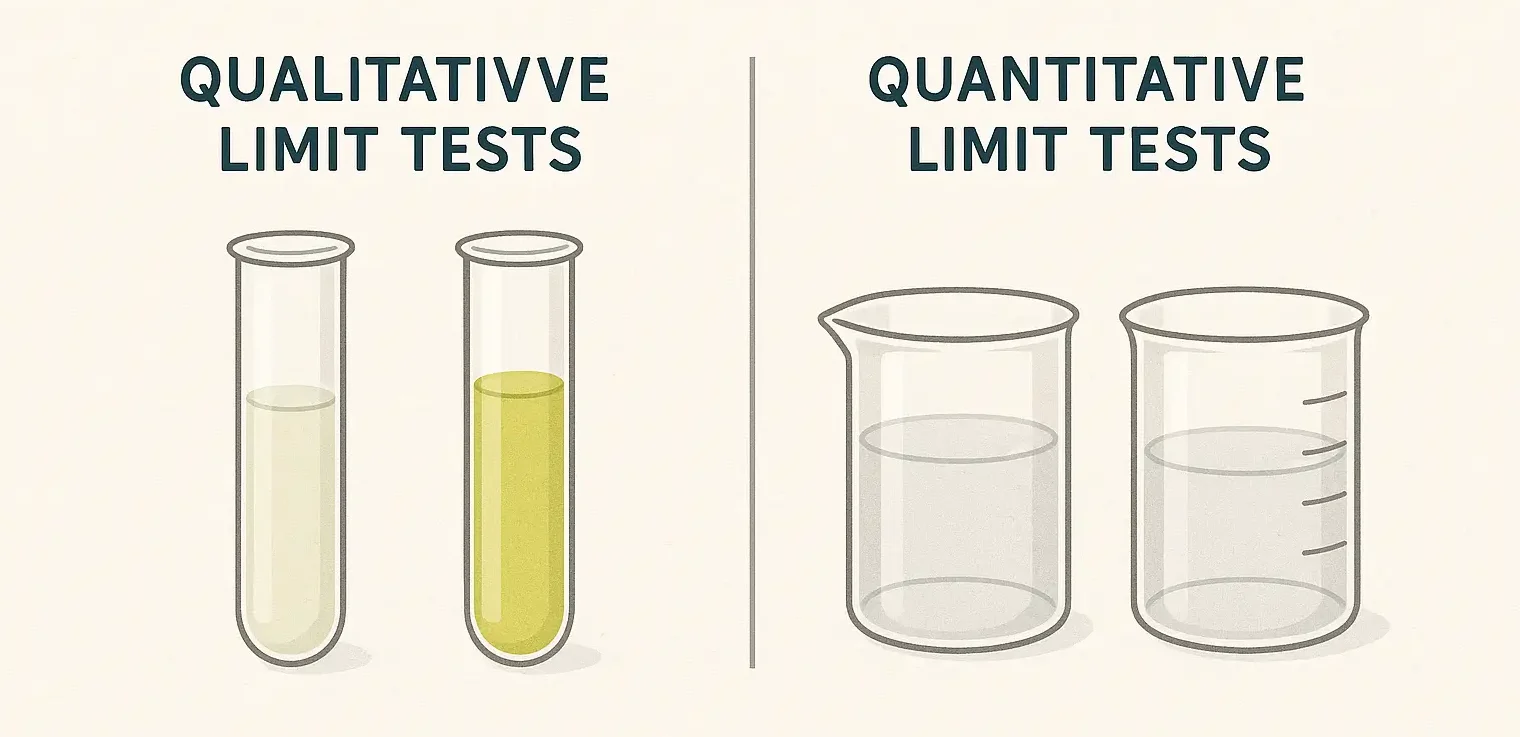
Qualitative Limit Tests:
- Provide a pass/fail result, indicating whether the impurity or substance is below or above the specified limit.
- Involves comparison with a reference standard, often using visual cues like color intensity or precipitate size.
-
Quantitative Limit Tests:
- Measure the actual concentration of the impurity or substance in the sample.
- Uses analytical techniques such as HPLC, GC, or AAS to accurately determine impurity levels.
Common Uses of Limit Test:
-
Heavy Metals:
- Ensures that toxic heavy metals (e.g., lead, cadmium, mercury, arsenic) are below acceptable limits to prevent harm.
-
Residual Solvents:
- Verifies that solvents used during synthesis or purification are within safe limits, as some solvents can be toxic or have undesirable effects.
-
Preservatives and Antioxidants:
- Confirms that the concentrations of preservatives or antioxidants are within acceptable ranges to ensure product stability and prevent contamination.
-
Microbial Contaminants:
- Assesses whether the microbial load is within safe limits to ensure the product’s safety and efficacy.
Advertisements